The Origins of AIDS (23 page)
Read The Origins of AIDS Online
Authors: Pepin

It is worth quoting at length from what Beheyt wrote about how his patients were infected:
The Congo contains various health institutions (maternity centres, hospitals, dispensaries, etc.) where every day local nurses give dozens, even hundreds, of injections in conditions such that sterilisation of the needle or the syringe is impossible. At the Dispensaire Antivénérien de la Croix-Rouge in Léopoldville, on average 300 injections are administered each day. The large number of patients and the small quantity of syringes available to the nursing staff preclude sterilisation by autoclave after each use. Used syringes are simply rinsed, first with water, then with alcohol and ether, and are ready for a new patient. The same type of procedure exists in all health institutions where a small number of nurses have to provide care to a large number of patients, with very scarce supplies. The syringe is used from one patient to the next, occasionally retaining small quantities of infectious blood, which are large enough to transmit the disease
.
52
In September 1955, the Croix-Rouge abruptly withdrew from running the STD clinics and the colony took over its clients, nurses and other workers. This must have involved some heated argument because in that year’s annual report it appears that the Croix-Rouge transferred responsibility for this activity to the Léopoldville Department of Hygiene following some mutual agreement, while at the receiving end the latter made it clear in its own annual report that
the running of the STD clinics had been dumped on it at very short notice
.
53
the running of the STD clinics had been dumped on it at very short notice
.
53
Nevertheless, the department did its job. By the end of 1957, 3,761 free women were registered, on whom a total of 26,123 screening examinations had been performed. The following year, 4,384 free women were registered, a rather substantial percentage given that the medical officers estimated that there were around 5,000 of them in the city. The number of injections, however, was drastically reduced. Long-acting
penicillin completely replaced arsenicals for patients with syphilis, and the indications for such treatment were tightened as physicians acknowledged that it was unnecessary to treat those who probably carried only a ‘serological scar’ due to a past episode of yaws or a past syphilis which had been adequately treated
.
54
penicillin completely replaced arsenicals for patients with syphilis, and the indications for such treatment were tightened as physicians acknowledged that it was unnecessary to treat those who probably carried only a ‘serological scar’ due to a past episode of yaws or a past syphilis which had been adequately treated
.
54
The department of health also ran the annual medical census of the whole population of Léopoldville, for which each and every inhabitant was examined summarily to look for sleeping sickness and
leprosy. This represented a substantial effort: between 148,584 (1949) and 322,198 (1958) individuals were examined, all to detect less than 100 cases of sleeping sickness and a few lepers annually. During the last years before independence, in a never-to-be-repeated population measure of the prevalence of symptomatic STDs, the health agents running the medical census required every adult male to drop his pants: of 99,446 men seen in 1958, 163 were found to have
gonorrhoea, 335 to have non-gonococcal urethritis (presumably, in retrospect,
chlamydia) while only 44 had a chancre suggestive of
syphilis.
47
,
55
leprosy. This represented a substantial effort: between 148,584 (1949) and 322,198 (1958) individuals were examined, all to detect less than 100 cases of sleeping sickness and a few lepers annually. During the last years before independence, in a never-to-be-repeated population measure of the prevalence of symptomatic STDs, the health agents running the medical census required every adult male to drop his pants: of 99,446 men seen in 1958, 163 were found to have
gonorrhoea, 335 to have non-gonococcal urethritis (presumably, in retrospect,
chlamydia) while only 44 had a chancre suggestive of
syphilis.
47
,
55
It is remarkable that throughout this period the incidence of other tropical diseases, for which injectable drugs were massively used in the rural areas, including those around Léopoldville, remained minimal in the Belgian Congo’s capital.
After 1930, there were generally fewer than 100 cases of sleeping sickness diagnosed each year, mostly in migrants from endemic areas, and similar numbers of cases of yaws. To some extent, this was a result of the systematic screening of migrants arriving in Léo so that a large proportion of sleeping sickness cases were identified and treated, which reduced the risk of transmission of the parasite within Léo. This was very good news for its large Belgian population as the risk of being bitten by an infectious tsetse fly was reduced accordingly. Yaws remained uncommon, reflecting an easier access to health care (treatment shortened the duration of infectiousness) and hygienic conditions which, even if far worse than those of the Europeans, were
healthier than in rural areas. In the African suburbs, water was readily available, not in each house but at a shared tap for several compounds
. Consequently, in Léo the treatment of STDs, especially ‘syphilis’, provided the best opportunity for the iatrogenic transmission of infectious agents through syringes and needles
.
After 1930, there were generally fewer than 100 cases of sleeping sickness diagnosed each year, mostly in migrants from endemic areas, and similar numbers of cases of yaws. To some extent, this was a result of the systematic screening of migrants arriving in Léo so that a large proportion of sleeping sickness cases were identified and treated, which reduced the risk of transmission of the parasite within Léo. This was very good news for its large Belgian population as the risk of being bitten by an infectious tsetse fly was reduced accordingly. Yaws remained uncommon, reflecting an easier access to health care (treatment shortened the duration of infectiousness) and hygienic conditions which, even if far worse than those of the Europeans, were
healthier than in rural areas. In the African suburbs, water was readily available, not in each house but at a shared tap for several compounds
. Consequently, in Léo the treatment of STDs, especially ‘syphilis’, provided the best opportunity for the iatrogenic transmission of infectious agents through syringes and needles
.
Less than 0.1% of the total population of the central chimpanzee inhabited the Belgian Congo, so it is unlikely that ‘patient zero’, the one who started the pandemic, lived there. However, Léo was the most dynamic city in the region, a commercial hub which attracted large numbers of migrants and traders
. A SIV
cpz
-infected cut hunter moving to the city or an HIV-1-infected trader wishing to spend some time in the capital would have to present himself at the STD clinic upon arrival, where he would receive treatment for
syphilis if his serological assay was positive, either because of prior syphilis or much more often because of prior yaws. Alternatively, one can imagine that a first HIV-infected free woman sexually infected by one of her patrons would be treated with IV drugs, also because of a positive serology.
. A SIV
cpz
-infected cut hunter moving to the city or an HIV-1-infected trader wishing to spend some time in the capital would have to present himself at the STD clinic upon arrival, where he would receive treatment for
syphilis if his serological assay was positive, either because of prior syphilis or much more often because of prior yaws. Alternatively, one can imagine that a first HIV-infected free woman sexually infected by one of her patrons would be treated with IV drugs, also because of a positive serology.
Once the virus was introduced within the Léopoldville–Brazzaville conurbation, it would have found an extraordinary opportunity for its amplification through non-sterile syringes and needles at the Dispensaire Antivénérien in the
Barumbu district of Léo-Est. There, the caseload of patients was extreme due to the obsession of local physicians with treating anybody with a positive ‘
syphilis’ serology. Iatrogenic transmission of another blood-borne virus,
HBV, was well documented in 1951–2 to have occurred as a consequence of the inadequate sterilisation of injection equipment. It does not take much imagination to deduce that if HBV was transmitted iatrogenically, the same must have occurred with SIV
cpz
/HIV-1 once it was introduced into the cohort of patients treated for presumed STDs at the same Dispensaire Antivénérien. Many of the iatrogenically infected cases would have been free women who had concomitant sexual relationships with several men: it was indeed
the perfect storm
.
Barumbu district of Léo-Est. There, the caseload of patients was extreme due to the obsession of local physicians with treating anybody with a positive ‘
syphilis’ serology. Iatrogenic transmission of another blood-borne virus,
HBV, was well documented in 1951–2 to have occurred as a consequence of the inadequate sterilisation of injection equipment. It does not take much imagination to deduce that if HBV was transmitted iatrogenically, the same must have occurred with SIV
cpz
/HIV-1 once it was introduced into the cohort of patients treated for presumed STDs at the same Dispensaire Antivénérien. Many of the iatrogenically infected cases would have been free women who had concomitant sexual relationships with several men: it was indeed
the perfect storm
.
Those free women infected parenterally could then transmit the virus sexually to some of their regular clients, who in turn infected other sex workers, or later other women, eventually allowing the virus to move out of the sexual core group. This second part of the amplification
process, this time sexual, could proceed at a much faster pace when, during the chaotic years that followed the country’s accession to independence in 1960, the face of Léopoldville changed abruptly, with massive migrations, high unemployment rates and the emergence of a different type of prostitution in which some women might entertain up to 1,000 clients per year
.
process, this time sexual, could proceed at a much faster pace when, during the chaotic years that followed the country’s accession to independence in 1960, the face of Léopoldville changed abruptly, with massive migrations, high unemployment rates and the emergence of a different type of prostitution in which some women might entertain up to 1,000 clients per year
.
10
The other human immunodeficiency viruses
HIV-2 and Guiné Portuguesa
The other human immunodeficiency viruses
Although they contribute little to the overall burden of AIDS in the world, the other HIVs (HIV-1 groups O, N and P, and HIV-2) can provide useful insight into the events that led to the emergence of pandemic HIV-1 group M. How was it possible for
HIV-2, a different virus that originated from a different simian host, to spread in a different region of Africa at roughly the same time (give or take a few decades) as HIV-1, only to disappear quietly thereafter? And why was HIV-1 group M so successful compared to the others?
HIV-1 groups O, N and PHIV-2, a different virus that originated from a different simian host, to spread in a different region of Africa at roughly the same time (give or take a few decades) as HIV-1, only to disappear quietly thereafter? And why was HIV-1 group M so successful compared to the others?
Highly divergent strains of HIV-1 were described in the 1990s. The first, now known as HIV-1 group O (‘O’ for outlier), has only 50–65% homology in nucleotide sequences compared to HIV-1 group M, which is why it is considered as a different ‘group’ rather than a different ‘
subtype’ (subtypes differ by about 20%; in other words, they have 80% homology). The original isolates of HIV-1 group O had been obtained from two Cameroonians living in
Belgium, a young woman and her husband. Additional cases were documented among Cameroonians living in
France, and in Cameroon itself. Further studies confirmed that Cameroon was the epicentre of HIV-1 group O, where it accounted for 2% of all HIV-1 infections, versus 1% in adjacent
Gabon and
Nigeria. A few cases were found in other African countries. Within Cameroon, regional variations were noted, with group O representing 6% of all HIV-1 positive sera in
Yaoundé but only 1% in northern provinces. When stored sera were tested, group O represented 21% of all HIV-1 positive sera in 1986–8, 9% in 1989–91, 3% in 1994–5 and only 1% in 1997–8. It then remained rather stable at 1–2%.
1
–
7
subtype’ (subtypes differ by about 20%; in other words, they have 80% homology). The original isolates of HIV-1 group O had been obtained from two Cameroonians living in
Belgium, a young woman and her husband. Additional cases were documented among Cameroonians living in
France, and in Cameroon itself. Further studies confirmed that Cameroon was the epicentre of HIV-1 group O, where it accounted for 2% of all HIV-1 infections, versus 1% in adjacent
Gabon and
Nigeria. A few cases were found in other African countries. Within Cameroon, regional variations were noted, with group O representing 6% of all HIV-1 positive sera in
Yaoundé but only 1% in northern provinces. When stored sera were tested, group O represented 21% of all HIV-1 positive sera in 1986–8, 9% in 1989–91, 3% in 1994–5 and only 1% in 1997–8. It then remained rather stable at 1–2%.
1
–
7
To some extent, this decreasing proportion in the overall burden of HIV-1 infections was mistaken and reflected changes in the quality of
diagnostic assays used to sort out group O from group M. But it is also possible that, over the last two decades, HIV-1 group O proved less transmissible than HIV-1 group M, as suggested by its lack of success in spreading outside its central African epicentre. Among more than 10,000 new cases of HIV infection diagnosed in
France between 2003 and 2006, only twelve corresponded to HIV-1 group O (nine among Cameroonian migrants, one in a
Chadian and two in French nationals). In the laboratory, HIV-1 group O is less ‘fit’ than group M: it replicates less efficiently in cultures with lymphocytes, which may explain its lower transmissibility.
8
–
10
diagnostic assays used to sort out group O from group M. But it is also possible that, over the last two decades, HIV-1 group O proved less transmissible than HIV-1 group M, as suggested by its lack of success in spreading outside its central African epicentre. Among more than 10,000 new cases of HIV infection diagnosed in
France between 2003 and 2006, only twelve corresponded to HIV-1 group O (nine among Cameroonian migrants, one in a
Chadian and two in French nationals). In the laboratory, HIV-1 group O is less ‘fit’ than group M: it replicates less efficiently in cultures with lymphocytes, which may explain its lower transmissibility.
8
–
10
Using the same approaches as for HIV-1 group M, the past dynamics of HIV-1 group O were reconstructed. Remarkably, the most recent common ancestor of all group O isolates was dated around 1920, much the same as for group M but with a wide confidence interval (1890–1940). It is thought that all cases of group O infections resulted from a single cross-species transmission, following which the growth of the infected population was slower than for group M, doubling about every six years. By the late 1990s, of the half million Cameroonians who were HIV-infected, about 7,500 were infected with group O. As could be expected from this limited number of infected individuals, HIV-1 group O displays less
genetic diversity than group M, with only three or
four subtypes identified so far.
11
–
13
genetic diversity than group M, with only three or
four subtypes identified so far.
11
–
13
HIV-1 group N (‘N’ for non-M non-O, or new), which was isolated for the first time in 1995 from a Cameroonian with AIDS, had even less success in spreading among humans. Up to now, only thirteen cases have been documented, all in Cameroonians. This may be an underestimate since it is not easy to sort out group N from group M through serologic tests. Two such cases occurred within a couple, indicating some heterosexual transmission. Nucleotide sequences of group N are similar to those obtained from SIV
cpz
-infected
P.t. troglodytes
chimps from the same area of southern Cameroon. Group N isolates represent a single lineage with low diversity, perhaps because its introduction in human populations was more recent than groups M and O. The most recent common ancestor of group N HIV-1 isolates has been dated around 1963 (confidence interval: 1948–77).
14
–
17
cpz
-infected
P.t. troglodytes
chimps from the same area of southern Cameroon. Group N isolates represent a single lineage with low diversity, perhaps because its introduction in human populations was more recent than groups M and O. The most recent common ancestor of group N HIV-1 isolates has been dated around 1963 (confidence interval: 1948–77).
14
–
17
In
phylogenetic trees, both groups M and N lie within the radiation of SIV
cpz
isolates obtained from the
P.t. troglodytes
chimpanzee (
Figures 1
,
2
and
3
) and clearly originated from this same simian host. In contrast, the exact source of group O, the outlier, remained uncertain for some time.
The virus most closely related to group O is the recently described virus of gorillas, SIV
gor
. It seems likely that gorillas, like their human cousins, were infected from chimpanzees. Given the geographic distribution of groups O and N, there is little doubt that their cross-species transmission event, from ape to man, occurred in Cameroon.
18
,
19
phylogenetic trees, both groups M and N lie within the radiation of SIV
cpz
isolates obtained from the
P.t. troglodytes
chimpanzee (
Figures 1
,
2
and
3
) and clearly originated from this same simian host. In contrast, the exact source of group O, the outlier, remained uncertain for some time.
The virus most closely related to group O is the recently described virus of gorillas, SIV
gor
. It seems likely that gorillas, like their human cousins, were infected from chimpanzees. Given the geographic distribution of groups O and N, there is little doubt that their cross-species transmission event, from ape to man, occurred in Cameroon.
18
,
19
Recently, a new group of HIV-1, group P, has been identified following the isolation of a peculiar strain from (again) a Cameroonian living in
France. Phylogenetic analyses showed that HIV-1 group P is closest to SIV
gor
, and actually closer to SIV
gor
than HIV-1 group O is. The patient herself recalled no exposure to apes and presumably acquired the infection sexually from an infected man. At some point an initial transmission occurred from gorillas to humans, followed by limited inter-human spread. As with group O, the true source of the virus might be the chimpanzee, which could have infected humans and gorillas independently, or infected gorillas which later infected humans. A second case of group P infection has been identified by a diagnostics company in a patient hospitalised in Yaoundé
.
20
France. Phylogenetic analyses showed that HIV-1 group P is closest to SIV
gor
, and actually closer to SIV
gor
than HIV-1 group O is. The patient herself recalled no exposure to apes and presumably acquired the infection sexually from an infected man. At some point an initial transmission occurred from gorillas to humans, followed by limited inter-human spread. As with group O, the true source of the virus might be the chimpanzee, which could have infected humans and gorillas independently, or infected gorillas which later infected humans. A second case of group P infection has been identified by a diagnostics company in a patient hospitalised in Yaoundé
.
20
These findings about HIV-1 groups O, N and P have at least two implications. First, it shows that other SIV
cpz
isolates crossed the species barrier in the same geographic region where HIV-1 group M emerged in human populations. In the case of HIV-1 group O this seemed to have happened at roughly the same time as for group M, around 1920, while with group N it was more recent
. This supports the idea that cross-species transmissions from chimps to man might have gone on for hundreds of years without triggering a recognisable pandemic and leading only to epidemiological dead ends. One case in point is the HIV-1 group O-infected
Norwegian sailor who, in the 1960s, transmitted the virus to his wife who herself infected their child, without any further cases outside this nuclear family. Second, it suggests that one of the reasons behind the dramatic spread of HIV-1 group M might be intrinsic to this specific strain: compared to group O and to
HIV-2, HIV-1 group M is better at infecting lymphocytes, which increases its capacity to be transmitted sexually or otherwise from one human to another
.
cpz
isolates crossed the species barrier in the same geographic region where HIV-1 group M emerged in human populations. In the case of HIV-1 group O this seemed to have happened at roughly the same time as for group M, around 1920, while with group N it was more recent
. This supports the idea that cross-species transmissions from chimps to man might have gone on for hundreds of years without triggering a recognisable pandemic and leading only to epidemiological dead ends. One case in point is the HIV-1 group O-infected
Norwegian sailor who, in the 1960s, transmitted the virus to his wife who herself infected their child, without any further cases outside this nuclear family. Second, it suggests that one of the reasons behind the dramatic spread of HIV-1 group M might be intrinsic to this specific strain: compared to group O and to
HIV-2, HIV-1 group M is better at infecting lymphocytes, which increases its capacity to be transmitted sexually or otherwise from one human to another
.
Just a few years after their discovery of HIV-1 as the aetiological agent of AIDS, a different virus, soon to be named HIV-2, was isolated by the
same researchers at the
Institut Pasteur from two AIDS patients, one from Guinea-Bissau and the other from Cape Verde.
These observations were extended to a larger group of thirty patients recruited in
Lisbon with varying degrees of immunosuppression (seventeen with full-blown AIDS), all but two of whom originated from Guinea-Bissau or Cape Verde
. This virus had only 30–40% homology with HIV-1 for most of its genes, hence its designation as a different virus rather than just another group of HIV-1. HIV-2 is not just a medical curiosity but illuminates part of the history of HIV-1, because whatever factors were instrumental in the emergence of HIV-1 in central Africa must have existed as well in West Africa, a few thousand kilometres away.
21
–
22
same researchers at the
Institut Pasteur from two AIDS patients, one from Guinea-Bissau and the other from Cape Verde.
These observations were extended to a larger group of thirty patients recruited in
Lisbon with varying degrees of immunosuppression (seventeen with full-blown AIDS), all but two of whom originated from Guinea-Bissau or Cape Verde
. This virus had only 30–40% homology with HIV-1 for most of its genes, hence its designation as a different virus rather than just another group of HIV-1. HIV-2 is not just a medical curiosity but illuminates part of the history of HIV-1, because whatever factors were instrumental in the emergence of HIV-1 in central Africa must have existed as well in West Africa, a few thousand kilometres away.
21
–
22
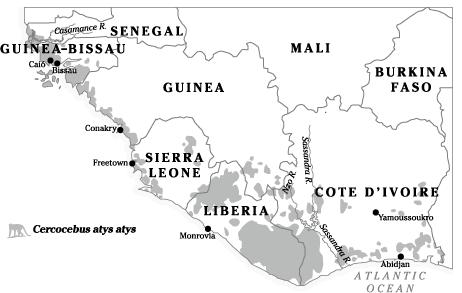
The source of HIV-2 was identified as the sooty mangabey
(
Cercocebus atys atys
), a small monkey which inhabits parts of coastal West Africa that correspond closely to the geographical distribution of HIV-2 (
Map 7
). This conclusion was based on the sequencing of simian viruses called SIV
smm
(smm for sooty mangabey monkey), isolated from this primate, which revealed a high degree of similarity with human HIV-2 isolates. SIV
smm
does not cause AIDS in its natural sooty mangabey hosts despite replicating at high levels, but causes disease when transferred to other species, especially the macaques, through cage infections. Presumably, when a given SIV has infected a given species
of monkey or ape for a long time, individuals who were more susceptible to the pathogenic effect of the virus, regardless of the underlying mechanisms, were preferentially removed from the population by death so that eventually the species became relatively resistant to the ill effects of this specific virus. One wonders whether the recent decrease in HIV-1 prevalence in some countries of East and southern Africa might to some extent reflect a similar process of natural selection in human populations.
23
–
27
(
Cercocebus atys atys
), a small monkey which inhabits parts of coastal West Africa that correspond closely to the geographical distribution of HIV-2 (
Map 7
). This conclusion was based on the sequencing of simian viruses called SIV
smm
(smm for sooty mangabey monkey), isolated from this primate, which revealed a high degree of similarity with human HIV-2 isolates. SIV
smm
does not cause AIDS in its natural sooty mangabey hosts despite replicating at high levels, but causes disease when transferred to other species, especially the macaques, through cage infections. Presumably, when a given SIV has infected a given species
of monkey or ape for a long time, individuals who were more susceptible to the pathogenic effect of the virus, regardless of the underlying mechanisms, were preferentially removed from the population by death so that eventually the species became relatively resistant to the ill effects of this specific virus. One wonders whether the recent decrease in HIV-1 prevalence in some countries of East and southern Africa might to some extent reflect a similar process of natural selection in human populations.
23
–
27

Map 7
Historical range of the sooty mangabey (
Cercocebus atys atys
) in West Africa.
Historical range of the sooty mangabey (
Cercocebus atys atys
) in West Africa.
SIV
smm
and HIV-2 sequences from animals and humans originating from the same geographic areas were found to be most related, which implies local activities as the route of transmission. In Liberia and Sierra Leone, 22% of free-living sooty mangabeys were infected with SIV
smm
, compared to only 4% of those kept as household pets, most of which had been removed from their native troops as infants.
Among sooty mangabeys in the
Taï Forest of Ivory Coast, SIV
smm
prevalence was even higher at 59%.
Because of hunting and destruction of their habitat, sooty mangabeys are now extinct in Senegal, Guinea-Bissau and parts of Guinea, while substantial populations remain in Sierra Leone, Liberia and Ivory Coast.
28
–
29
smm
and HIV-2 sequences from animals and humans originating from the same geographic areas were found to be most related, which implies local activities as the route of transmission. In Liberia and Sierra Leone, 22% of free-living sooty mangabeys were infected with SIV
smm
, compared to only 4% of those kept as household pets, most of which had been removed from their native troops as infants.
Among sooty mangabeys in the
Taï Forest of Ivory Coast, SIV
smm
prevalence was even higher at 59%.
Because of hunting and destruction of their habitat, sooty mangabeys are now extinct in Senegal, Guinea-Bissau and parts of Guinea, while substantial populations remain in Sierra Leone, Liberia and Ivory Coast.
28
–
29
Molecular studies revealed that HIV-2 can be divided into eight groups, defined according to their degree of
genetic diversity. Only groups A and B managed to spread between humans while groups C to H represent individual human cases documented in Liberia, Sierra Leone and Ivory Coast.
Each group represents at least one distinct cross-species transmission event, from the sooty mangabey to man.
In Guinea-Bissau and The Gambia, only HIV-2 group A is found. HIV-2 group B has been detected mostly in and around Ivory Coast. Sierra Leone, the country with the highest diversity of HIV-2 groups among humans, also has the lowest HIV-2 prevalence, at 0.02%. The 1,000-fold higher SIV
smm
prevalence among sooty mangabeys in the same country implies that inter-human transmission following an initial cross-species event was generally ineffective. But cross-species transmission of viruses from sooty mangabeys might have been more common than from chimpanzees, because the former live closer to humans and can be domesticated as pets
.
29
genetic diversity. Only groups A and B managed to spread between humans while groups C to H represent individual human cases documented in Liberia, Sierra Leone and Ivory Coast.
Each group represents at least one distinct cross-species transmission event, from the sooty mangabey to man.
In Guinea-Bissau and The Gambia, only HIV-2 group A is found. HIV-2 group B has been detected mostly in and around Ivory Coast. Sierra Leone, the country with the highest diversity of HIV-2 groups among humans, also has the lowest HIV-2 prevalence, at 0.02%. The 1,000-fold higher SIV
smm
prevalence among sooty mangabeys in the same country implies that inter-human transmission following an initial cross-species event was generally ineffective. But cross-species transmission of viruses from sooty mangabeys might have been more common than from chimpanzees, because the former live closer to humans and can be domesticated as pets
.
29
Epidemiological studies uncovered that the distribution of HIV-2 was largely limited to West Africa and that its epicentre was Guinea-Bissau, a tiny country that became independent only in 1974 after a protracted liberation war against the Portuguese dictatorship, where HIV-2
managed to infect 9% of adults. A lower prevalence (less than 2.5%) was found in other West African countries: Senegal
, The Gambia
,
Cape Verde,
Guinea, Liberia, Sierra Leone, Ivory Coast
,
Burkina
Faso, Ghana,
Mali and
Nigeria. A few cases were documented in distant countries that had had a colonial link with Portugal:
Angola,
Mozambique and
India
.
managed to infect 9% of adults. A lower prevalence (less than 2.5%) was found in other West African countries: Senegal
, The Gambia
,
Cape Verde,
Guinea, Liberia, Sierra Leone, Ivory Coast
,
Burkina
Faso, Ghana,
Mali and
Nigeria. A few cases were documented in distant countries that had had a colonial link with Portugal:
Angola,
Mozambique and
India
.
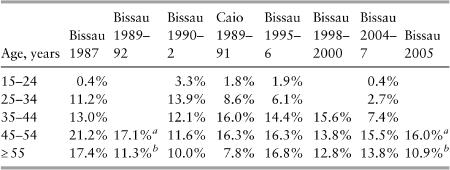
In Guinea-Bissau, an initial survey conducted in Bissau in 1987 revealed a HIV-2 prevalence of 8.9% amongst adults but with a marked age gradient, 20% of those aged forty and over being infected. HIV-1 was absent. Even though HIV-2 does cause AIDS, it is less pathogenic than HIV-1, increasing adult mortality by a factor of two to three while HIV-1 increases mortality ten-fold. In other words, HIV-2 is compatible with prolonged survival in a large portion (perhaps even the majority) of infected individuals, who will ultimately die of something else, in contrast with HIV-1 which kills nearly all untreated subjects within fifteen years. The age distribution of HIV-2, so different from that of HIV-1 elsewhere in Africa, was initially thought to reflect this low mortality and the effect of cumulative exposure over a long period of time.
30
–
32
30
–
32
Subsequently, HIV-2 was shown to result in a lower degree of
viraemia than HIV-1 and a lesser genital shedding in semen and cervical secretions. As a consequence, HIV-2 is less transmissible than HIV-1, both sexually and from mother to child. This raised the question of how, if the virus is poorly transmissible, prevalence could have reached such a high level in the first place.
33
–
35
viraemia than HIV-1 and a lesser genital shedding in semen and cervical secretions. As a consequence, HIV-2 is less transmissible than HIV-1, both sexually and from mother to child. This raised the question of how, if the virus is poorly transmissible, prevalence could have reached such a high level in the first place.
33
–
35
Serial surveys showed that the infection was so rare among younger people that cumulative exposure could not mathematically explain the high prevalence among the elderly (
Table 1
). This corresponded rather to what epidemiologists call a ‘cohort effect’: something peculiar happened to the cohort of individuals born before 1962, which did not apply to those born since. Research then focused on older people. Among women aged fifty and over, HIV-2 was associated with having had sex with a white man, possibly a proxy for prostitution. It was hypothesised that this cohort effect was related to changes in sexual activity (higher promiscuity, more commercial sex) during the 1963–74 liberation war, with the same people becoming less promiscuous after the war came to an end.
30
,
36
–
41
Table 1
). This corresponded rather to what epidemiologists call a ‘cohort effect’: something peculiar happened to the cohort of individuals born before 1962, which did not apply to those born since. Research then focused on older people. Among women aged fifty and over, HIV-2 was associated with having had sex with a white man, possibly a proxy for prostitution. It was hypothesised that this cohort effect was related to changes in sexual activity (higher promiscuity, more commercial sex) during the 1963–74 liberation war, with the same people becoming less promiscuous after the war came to an end.
30
,
36
–
41
Bissau-Guineans had fought on both sides. Some were
guerilleros
of the liberation movement, which controlled rural areas whose size
increased as the war progressed, while others had been conscripted into the colonial army, alongside Portuguese soldiers, which controlled the cities. There were problems with this ‘promiscuous soldier’ theory. First, the initial study showed no associations between HIV-2 infection and either having served in the Portuguese army or the duration of military service. Second, the underlying idea that sexual activity was higher during wartime remained unproven. The conflict in Guinea-Bissau had been a guerrilla war, with fighters from the liberation movement constantly moving to avoid the better armed Portuguese troops and their small aeroplanes. While soldiers stationed in peaceful countries certainly tend to frequent sex workers and acquire STDs, there is little evidence that such prostitution occurs close to combat areas.
Recent experiences in Ivory Coast and the DRC revealed, if anything, decreasing HIV-1 prevalence after a period of conflict. When survival becomes the main concern, there is less time and energy for sex, and in disciplined armies rapes are too uncommon to have a measurable effect on HIV transmission. Then Portuguese doctors tested almost 2,000 blood donors who had served in the colonial army in Guinea-Bissau (and who would have been more exposed to prostitutes than their guerrilla opponents): not a single one of them was HIV-2-infected. Either the Portuguese soldiers were extremely virtuous or they were resistant to HIV-2 infection – or the hypothesis was wrong
.
36
,
42
–
44
guerilleros
of the liberation movement, which controlled rural areas whose size
increased as the war progressed, while others had been conscripted into the colonial army, alongside Portuguese soldiers, which controlled the cities. There were problems with this ‘promiscuous soldier’ theory. First, the initial study showed no associations between HIV-2 infection and either having served in the Portuguese army or the duration of military service. Second, the underlying idea that sexual activity was higher during wartime remained unproven. The conflict in Guinea-Bissau had been a guerrilla war, with fighters from the liberation movement constantly moving to avoid the better armed Portuguese troops and their small aeroplanes. While soldiers stationed in peaceful countries certainly tend to frequent sex workers and acquire STDs, there is little evidence that such prostitution occurs close to combat areas.
Recent experiences in Ivory Coast and the DRC revealed, if anything, decreasing HIV-1 prevalence after a period of conflict. When survival becomes the main concern, there is less time and energy for sex, and in disciplined armies rapes are too uncommon to have a measurable effect on HIV transmission. Then Portuguese doctors tested almost 2,000 blood donors who had served in the colonial army in Guinea-Bissau (and who would have been more exposed to prostitutes than their guerrilla opponents): not a single one of them was HIV-2-infected. Either the Portuguese soldiers were extremely virtuous or they were resistant to HIV-2 infection – or the hypothesis was wrong
.
36
,
42
–
44
To reconstruct the past dynamics of HIV-2 in Guinea-Bissau, the same approaches were used as for HIV-1. Archival samples obtained
in rural areas during a 1980
yellow fever survey were tested. Out of 1,234 specimens, eleven were HIV-2-reactive. Prevalence increased with age and 6% of those more than forty-five years of age were infected. These are the oldest specimens from that country. HIV-2 antibodies were found in a few specimens obtained in the late 1960s or early 1970s in Ivory Coast,
Mali,
Nigeria,
Senegal and
Gabon, but not in samples from
Liberia,
Sierra Leone,
Togo,
Chad,
Niger and
Ghana.
45
–
47
in rural areas during a 1980
yellow fever survey were tested. Out of 1,234 specimens, eleven were HIV-2-reactive. Prevalence increased with age and 6% of those more than forty-five years of age were infected. These are the oldest specimens from that country. HIV-2 antibodies were found in a few specimens obtained in the late 1960s or early 1970s in Ivory Coast,
Mali,
Nigeria,
Senegal and
Gabon, but not in samples from
Liberia,
Sierra Leone,
Togo,
Chad,
Niger and
Ghana.
45
–
47
Cases of HIV-2 AIDS, confirmed by serology, were recognised retrospectively, first in a Portuguese patient who had lived in Guinea-Bissau between 1956 and 1966 and died in 1979. Another case was diagnosed in 1978 in a Portuguese man who had served in the colonial army in
Angola between 1968 and 1974 and then travelled between Angola and
Mozambique. A Portuguese couple developed AIDS in the early 1980s, and HIV-2 infection could be traced to the man having served in the colonial army in Guinea-Bissau between 1966 and 1969. A Portuguese woman who had received a blood transfusion in Guinea-Bissau in 1967 remained asymptomatic twenty-seven years later, albeit with decreased CD4 lymphocyte counts. These case reports demonstrated that HIV-2 had been present in Guinea-Bissau and other Portuguese colonies at least since the 1960s, and that the incubation period between infection and AIDS was longer than with HIV-1.
48
–
51
Angola between 1968 and 1974 and then travelled between Angola and
Mozambique. A Portuguese couple developed AIDS in the early 1980s, and HIV-2 infection could be traced to the man having served in the colonial army in Guinea-Bissau between 1966 and 1969. A Portuguese woman who had received a blood transfusion in Guinea-Bissau in 1967 remained asymptomatic twenty-seven years later, albeit with decreased CD4 lymphocyte counts. These case reports demonstrated that HIV-2 had been present in Guinea-Bissau and other Portuguese colonies at least since the 1960s, and that the incubation period between infection and AIDS was longer than with HIV-1.
48
–
51
Molecular clock analyses estimated that the most recent common ancestors for HIV-2 group A existed around 1940, and for HIV-2 group B around 1945, two decades later than for HIV-1 group M. Using mathematical models, it was estimated that in Caio, a high-prevalence village of Guinea-Bissau, a period of exponential growth occurred between 1955 and 1970. A more recent study using slightly different methods dated the most recent common ancestors to 1932 (group A) and 1935 (group B). These findings are in line with the notion that the
sooty mangabey became extinct in Guinea Bissau a few decades ago, so that the initial
cross-species event must have occurred earlier.
52
–
53
sooty mangabey became extinct in Guinea Bissau a few decades ago, so that the initial
cross-species event must have occurred earlier.
52
–
53
Other books
Tails and Teapots by Misa Izanaki
Cherryh, C J - Alliance-Union 08 by Cyteen Trilogy V1 1 html
Bigger (The Nicky Beets series) by Mayes, Erin
Reckless by Kimberly Kincaid
SHUDDERVILLE FIVE by Zabrisky, Mia
Spare and Found Parts by Sarah Maria Griffin
A Tiger for Malgudi by R. K. Narayan
The Guv'nor by Lenny McLean
The Holy City by Patrick McCabe
The Air War by Adrian Tchaikovsky