Your Inner Fish: A Journey Into the 3.5-Billion-Year History of the Human Body (22 page)
Read Your Inner Fish: A Journey Into the 3.5-Billion-Year History of the Human Body Online
Authors: Neil Shubin

Our sense of smell allows us to discriminate among five thousand to ten thousand odors. Some people can detect the odor molecules in a green bell pepper at a concentration of less than one part per trillion. That is like picking out one grain of sand from a mile-long beach. How do we do that?
What we perceive as a smell is our brain’s response to a cocktail of molecules floating in the air. The molecules that we ultimately register as an odor are tiny, light enough to be suspended in the air. As we breathe or sniff, we suck these odor molecules into our nostrils. From there, the odor molecules go to an area behind our nose where they are trapped by the mucous lining of our nasal passages. Inside this lining is a patch of tissue containing millions of nerve cells, each with little projections into the mucous membrane. When the molecules in the air bind to the nerve cells, signals are sent to our brain. Our brain records these signals as a smell.
The molecular part of smelling works like a lock-and-key mechanism. The lock is the odor molecule; the key is the receptor on the nerve cell. A molecule captured by the mucous membranes in our nose interacts with a receptor on the nerve cell. Only when the molecule attaches to the receptor is a signal sent to our brain. Each receptor is tuned to a different kind of molecule, so a particular odor might involve lots of molecules and, accordingly, lots of receptors sending signals to our brains.
The best analogy for smell comes from music: a chord. A chord is made up of several notes acting together as one. In the same way, an odor is the product of signals from lots of receptors keyed to different odor molecules. Our brain perceives these different impulses as one smell.
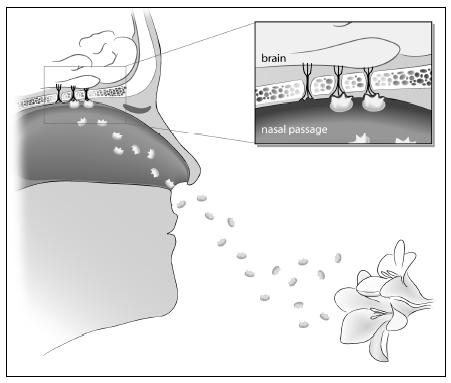
Molecules (enlarged many, many times) from a flower waft through the air. These molecules attach to receptors inside the lining of our nasal cavities. Once the molecules attach, a signal is sent to our brain. Each smell is composed of many different molecules attaching to different receptors. Our brain integrates these signals as we perceive a smell.
As in fish, amphibians, reptiles, mammals, and birds, much of our sense of smell is housed inside our skull. Like the other animals, we have one or more holes through which we bring air inside, and then a set of specialized tissues where the chemicals in the air can interact with neurons. We can trace the patterns of these holes, spaces, and membranes from fish to man and find a general pattern. The most primitive living animals with skulls, jawless fish such as lampreys and hagfish, have a single nostril that leads to a sac inside the skull. Water goes into this blind sac, and it is there that smelling takes place; the main difference from us being that lampreys and hagfish extract odors from water instead of air. Our closest fish relatives have an arrangement somewhat like ours: the water enters a nostril and ultimately goes to a cavity linked with the mouth. Fish like lungfish or
Tiktaalik
have two kinds of nostrils: an external one and an internal one. In this, they are a lot like us. Sit with your mouth closed and breathe. Air enters an external nostril and travels through your nasal cavities to enter the back of your throat via internal passageways. Our fish ancestors had internal and external nostrils, too, and to nobody’s surprise these are the same fish that have arm bones and other features in common with us.
Our sense of smell contains a deep record of our history as fish, amphibians, and mammals. A major breakthrough in understanding this occurred in 1991 when Linda Buck and Richard Axel discovered the large family of genes that give us our sense of smell.
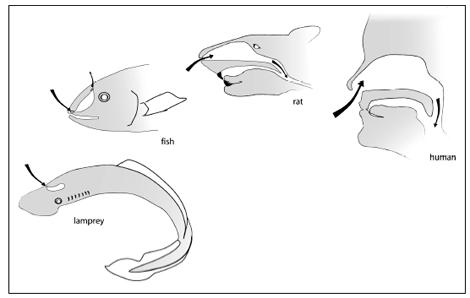
Nasal openings and the flow of odor molecules from jawless fish to man.
Buck and Axel used three major assumptions to design their experiments. First, they came up with a reasoned hypothesis, based on work done in other laboratories, about what the genes that make odor receptors might have looked like. Experiments showed that odor receptors have a characteristic structure with a number of molecular loops that help them convey information across a cell. This was a big clue, because Buck and Axel could then search the genome of a mouse for every gene that makes this structure. Second, they assumed that the genes for these receptors had to have a very specific activity—they should be active only in the tissues involved with smell. This makes sense: if something is involved in smelling, then it should be restricted to the tissues specialized for that purpose. Third—and this last was a big assumption—Axel and Buck reasoned that there wasn’t only one or even a small number of these genes, there had to be lots of them. This hypothesis was based on the fact that many different kinds of chemicals can stimulate different smells. If there was a one-to-one match between each chemical type and a receptor/ gene specialized for it, then there had to be many, many genes. But, given the data they had at the time, this needn’t have been true.
Buck and Axel’s three assumptions were borne out perfectly. They found genes that had the characteristic structure of the receptor they were looking for. They found that all of these genes were active only in the tissues involved in smelling, the olfactory epithelium. And finally, they found a huge number of these genes. It was a home run. Then, Buck and Axel discovered something truly astounding: fully 3 percent of our entire genome is devoted to genes for detecting different odors. Each of these genes makes a receptor for an odor molecule. For this work, Buck and Axel shared the Nobel Prize in 2004.
Following Buck and Axel’s success, people started fishing around for olfactory receptor genes in other species. It turns out that such genes are a living record of some major transitions in the history of life. Take the water-to-land transition, over 365 million years ago. There are two kinds of smelling genes: one is specialized for picking up chemical scents in the water, the other specialized for air. The chemical reaction between odor molecule and receptor is different in water and air, hence the need for slightly different receptors. As we’d expect, fish have water-based receptors in their nasal neurons, mammals and reptiles have air-based ones.
This discovery helps us make sense of the state of affairs in the most primitive fish alive on the planet today—the jawless fish such as lampreys and hagfish. It turns out that these creatures have, unlike more advanced fish and mammals, neither “air” nor “water” genes; rather, their receptors combine both types. The implication is clear: these primitive fish arose before the smelling genes split into two types.
Jawless fish reveal another very important point: they have a very small number of odor genes. Bony fish have more, and still more are seen in amphibians and reptiles. The number of odor genes has increased over time, from relatively few in primitive creatures such as jawless fish, to the enormous number seen in mammals. We mammals, with over a thousand of these genes, devote a huge part of our entire genetic apparatus just to smelling. Presumably, the more of these genes an animal has, the more acute its ability to discern different kinds of smells. In this light, our large number of odor genes makes sense—mammals are highly specialized smelling animals. Just think of what effective trackers dogs can be.
But where do all our extra odor genes come from? Did they just pop out of the blue? How this expansion happened seems obvious when we look at the structure of the genes. If you compare the odor genes of a mammal with the handful of odor genes in a jawless fish, the “extra” genes in mammals are all variations on a theme: they look like copies, albeit modified ones, of the genes in jawless fish. This means that our large number of odor genes arose by many rounds of duplication of the small number of genes present in primitive species.
This leads us to a paradox. Humans devote about 3 percent of our genome to odor genes, just like every other mammal. When geneticists looked at the structure of the human genes in more detail, they found a big surprise: fully three hundred of these thousand genes are rendered completely functionless by mutations that have altered their structure beyond repair. (Other mammals do use these genes.) Why have so many odor genes if so many of them are entirely useless?
Dolphins and whales, of all creatures, offer an insight to help us answer this question. Like all mammals, dolphins and whales have hair, breasts, and a three-boned middle ear. Their mammalian history is also recorded in their smelling genes: lacking fish-like water-specialized genes, cetaceans have mammalian air-specialized genes. The mammalian history of whales and dolphins is even written in the DNA of their odor perception apparatus. But there is an interesting puzzle: dolphins and whales no longer use their nasal passages to smell. What are these genes doing? The former nasal passage has been modified into a blowhole, which is used in breathing, not in smelling. This has had a remarkable effect on the smelling genes: all of a cetacean’s odor genes are present, but not one is functional.
What has happened to the smell genes of dolphins and whales also happens in many other species’ genes. Mutations crop up in genomes from generation to generation. If a mutation knocks out the function of a gene, the result can be dangerous, or even lethal. But what happens if a mutation knocks out the function of a gene that does nothing? There is a lot of mathematical theory that says the obvious: such mutations will just silently get passed on from generation to generation. This is exactly what appears to have happened in dolphins. Their smell genes are no longer needed, given the blowhole, so the mutations that knocked out their function just accumulate over time. The genes have no use, but they remain present in the DNA as silent records of evolution.
But humans do have a sense of smell, so why have so many of our odor genes been knocked out? Yoav Gilad and his colleagues answered this question by comparing genes among different primates. He found that primates that develop color vision tend to have large numbers of knocked-out smell genes. The conclusion is clear. We humans are part of a lineage that has traded smell for sight. We now rely on vision more than on smell, and this is reflected in our genome. In this trade-off, our sense of smell was deemphasized, and many of our olfactory genes became functionless.
We carry a lot of baggage in our noses—or, more precisely, in the DNA that controls our sense of smell. Our hundreds of useless olfactory genes are left over from mammal ancestors who relied more heavily on the sense of smell to survive. In fact, we can take these comparisons deeper still. Like photocopies that lose their fidelity as they are repeatedly copied, our olfactory genes get more dissimilar as we compare ourselves to successively more primitive creatures. Our genes are similar to primates’, less similar to other mammals’, less similar still to reptiles’, amphibians’, fishes’, and so on. That baggage is a silent witness to our past; inside our noses is a veritable tree of life.
CHAPTER NINE
