Your Inner Fish: A Journey Into the 3.5-Billion-Year History of the Human Body (19 page)
Read Your Inner Fish: A Journey Into the 3.5-Billion-Year History of the Human Body Online
Authors: Neil Shubin

The first people to see the earliest bodies in the fossil record had no idea what they were looking at. Between 1920 and 1960 really odd fossils started popping up from all around the world. In the 1920s and 1930s, Martin Gurich, a German paleontologist working in what is today Namibia, discovered a variety of impressions of what looked like animal bodies. Shaped like disks and plates, these things seemed unremarkable: they could have been primitive algae or jellyfish living in ancient seas.
In 1947, an Australian mining geologist named Reginald Sprigg happened upon a locality where the undersides of the rocks contained impressions of disks, ribbons, and fronds. Working around an abandoned mine in the Ediacara Hills of South Australia, Sprigg uncovered a collection of these fossils and described them dutifully. Over time, similar impressions became known from every continent of the world except Antarctica. Sprigg’s creatures seemed strange, but few people really cared about them.
The reason for the collective paleontological yawn was that these fossils were thought to come from the relatively young rocks of the Cambrian era, when many animal fossils with primitive bodies were already known. Sprigg’s and Gurich’s fossils sat relatively unnoticed, an assemblage of not terribly exciting, if weird, impressions from a period already well represented in the museum collections of the world.
In the mid-1960s, Martin Glaessner, a charismatic Austrian ex-pat living in Australia, changed all that. After comparing these rocks to those in other parts of the world, Glaessner showed that without a doubt these fossils were 15 million to 20 million years older than originally thought. They were no dull collection of impressions—rather, Gurich, Sprigg, and others were seeing the earliest bodies.
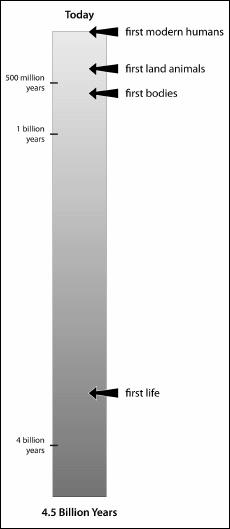
A timescale for events in the history of life. Notice the extremely long period of time during which there were no bodies on earth, only single-celled organisms living alone or in colonies.
These fossils came from the period known as the Precambrian, whose name literally means “Before Life.” Our understanding of the antiquity of life had just exploded. Paleontological curiosities became scientific jewels.
The Precambrian disks, ribbons, and fronds are clearly the oldest creatures with bodies. As we’d expect from other early animal fossils, they include representatives of some of the most primitive animals on the planet today: sponges and jellyfish. Other Precambrian fossils look like nothing known. We can tell that they are impressions of something with a body, but their patterns of blobs, stripes, and shapes match no living creature.
One message from this is very clear: creatures with many cells began to populate the seas of the planet by 600 million years ago. These creatures had well-defined bodies and weren’t just colonies of cells. They have patterns of symmetry that, in some cases, resemble those of living forms. As for those that cannot be compared directly with living forms, different parts of their bodies nevertheless have specialized structures. This implies that the Precambrian organisms had a level of biological organization that at the time was utterly new on the planet.
Evidence of these changes is seen not only in the fossil bodies but also in the rocks themselves. With the first bodies come the first trackways. Etched in the rocks are the first signs that creatures were actually crawling and squirming through the ooze. The earliest trackways, small ribbon-shaped scrapes in the ancient mud, show that some of these creatures with bodies were capable of relatively complicated motions. Not only did they have bodies with identifiable parts, but they were actually using them to move in new ways.
All of this makes total sense. We see the first bodies before we see the first body plans. We see the first primitive body plans before we see the first body plans with heads, and so on. Like the imaginary zoo we walked through in the first chapter, the rocks of the world are highly ordered.
As we said at the beginning of this section, we are after the when, how, and why of bodies. The Precambrian discoveries tell us the when. To see the how, and ultimately the why, we need to take a slightly different tack.
OUR OWN BODY OF EVIDENCE

A photo could never capture just how much of our bodies is to be found within those Precambrian disks, fronds, and ribbons. What could we humans, with all our complexity, ever share with impressions in rocks, particularly ones that look like crinkled jellyfish and squashed rolls of film?
The answer is profound and, when we see the evidence, inescapable: the “stuff” that holds us together—that makes our bodies possible—is no different from what formed the bodies of Gurich’s and Sprigg’s ancient impressions. In fact, the scaffolding of our entire body originated in a surprisingly ancient place: single-celled animals.
What holds a clump of cells together, whether they form a jellyfish or an eyeball? In creatures like us, that biological glue is astoundingly complicated; it not only holds our cells together, but also allows cells to communicate and forms much of our structure. The glue is not one thing; it is a variety of different molecules that connect and lie between our cells. At the microscopic level, it gives each of our tissues and organs its distinctive appearance and function. An eyeball looks different from a leg bone whether we look at it with the naked eye or under a microscope. In fact, much of the difference between a leg bone and an eye rests in the ways the cells and materials are arranged deep inside.
Every fall for the past several years, I have driven medical students crazy with just these concepts. Nervous first-year students must learn to identify organs by looking at random slides of tissue under a microscope. How do they do this?
The task is a little like figuring out what country you are in by looking at a street map of a small village. The task is doable, but we need the right clues. In organs, some of the best clues lie in the shape of cells and how they attach to one another; it is also important to be able to identify the stuff that lies between them. Tissues have all kinds of different cells, which attach to one another in different ways: some regions have strips or columns of cells; in others, cells are randomly scattered and loosely attached to one another. These areas, where cells are loosely packed, are often filled with materials that give each tissue its characteristic physical properties. For instance, the minerals that lie between bone cells determine the hardness of bone, whereas the looser proteins in the whites of our eyes make the wall of the eyeball more pliant.
Our students’ ability to identify organs from microscope slides, then, comes from knowing how cells are arranged and what lies between the cells. For us, there is a deeper meaning. The molecules that make these cellular arrangements possible are the molecules that make bodies possible. If there were no way to attach cells to one another, or if there were no materials between cells, there would be no bodies on the earth—just batches of cells. This means that the starting point for understanding how and why bodies arose is to see these molecules: the molecules that help cells stick together, the molecules that allow them to communicate with one another, and the substances that lie between cells.
To understand the relevance of this molecular structure to our bodies, let’s focus in detail on one part: our skeleton. Our skeleton is a powerful example of how tiny molecules can have a big impact on the structure of our body and exemplifies general principles that apply to all the body’s parts. Without skeletons, we would be formless masses of goo. Living on land would not be easy or even possible. So much of our basic biology and behavior is made possible by our skeleton that we often take it for granted. Every time we walk, play piano, inhale, or chew food we have our skeleton to thank.
A great analogy for the workings of our skeleton is a bridge. The strength of a bridge depends on the sizes, shapes, and proportions of its girders and cables. But also, importantly, the strength of the bridge depends on the microscopic properties of the materials from which it is made. The molecular structure of steel determines how strong it is and how far it will bend before breaking. In the same way, our skeleton’s strength is based on the sizes and shapes of our bones, but also on the molecular properties of our bones themselves.
Let’s go for a run to see how. As we jog along a path, our muscles contract, our back, arms, and legs move, and our feet push against the ground to move us forward. Our bones and joints function like a giant complex of levers and pulleys that make all that movement possible. Our body’s movements are governed by basic physics: our ability to run is in large part based on the size, shape, and proportions of our skeleton and the configuration of our joints. At this level, we look like a big machine. And like a machine, our design matches our functions. A world-class high jumper has different bone proportions from a champion sumo wrestler. The proportions of the legs of a rabbit or a frog, specialized to hop and jump, are different from those of a horse.
Now, let’s take a more microscopic view. Pop a slice of a femur under the microscope, and you will immediately see what gives bone its distinctive mechanical properties. The cells are highly organized in places, particularly on the outer rim of the bone. Some cells stick together, whereas others are separated. Between the separated cells are the materials that define the strength of bone. One of them is the rock, or crystal, known as hydroxyapatite, which we discussed in Chapter 4. Hydroxyapatite is hard the way concrete is: strong when compressed, less strong if twisted or bent. So, like a building made of bricks or concrete, bones are shaped so as to maximize their compressive functions and minimize twisting and bending, something Galileo recognized in the seventeenth century.
The other molecule found between our bone cells is the most common protein in the entire human body. If we magnify it 10,000 times with an electron microscope, we see something that looks like a rope consisting of bundles of little molecular fibers. This molecule, collagen, also has the mechanical properties of a rope. Rope is relatively strong when pulled, but it collapses when compressed; think of the two teams in a tug-of-war running toward the middle. Collagen, like rope, is strong when pulled but weak when the ends are pushed together.
Bone is composed of cells that sit in a sea of hydroxyapatite, collagen, and some other, less common molecules. Some cells stick together; other cells float inside these materials. The strength of bone is based on collagen’s strength when pulled, and on hydroxyapatite’s strength when compressed.
Cartilage, the other tissue in our skeleton, behaves somewhat differently. During our jog, it was the cartilage in our joints that provided the smooth surfaces where our bones glided against one another. Cartilage is a much more pliant tissue than bone; it can bend and smush as forces are applied to it. The smooth operation of the knee joint, as well as most of the other joints we used during our jog, depends on having relatively soft cartilage. When healthy cartilage is compressed it always returns to its native shape, like a kitchen sponge. During each step of our run, our entire body mass slams against the ground at some speed. Without these protective caps at our joints our bones would grind against one another: a very unpleasant and debilitating outcome of arthritis.
The pliability of cartilage is a property of its microscopic structure. The cartilage at our joints has relatively few cells, and these cells are separated by a lot of filling between them. As with bone, it is the properties of this interstitial filling that largely determine the mechanical properties of the cartilage.
Collagen fills much of the space between cartilage cells (as well as the cells of our other tissues). What really gives cartilage its pliancy is another kind of molecule, one of the most extraordinary in the whole body. This kind of molecule, called a proteoglycan complex, gives cartilage strength when squeezed or compressed. Shaped like a giant three-dimensional brush, with a long stem and lots of little branches, the proteoglycan complex is actually visible under a microscope. It has an amazing property relevant to our abilities to walk and move, thanks to the fact that the tiniest branches love to attach to water. A proteoglycan, then, is a molecule that actually swells up with water, filling up until it’s like a giant piece of Jell-O. Take this piece of gelatin, wrap collagen ropes in and around it, and you end up with a substance that is both pliant and somewhat resistant to tension. This, essentially, is cartilage. A perfect pad for our joints. The role of the cartilage cells is to secrete these molecules when the animal is growing and maintain them when the animal is not.