Coming of Age in the Milky Way (41 page)
Read Coming of Age in the Milky Way Online
Authors: Timothy Ferris
Tags: #Science, #Philosophy, #Space and time, #Cosmology, #Science - History, #Astronomy, #Metaphysics, #History

This was not the view, however, taken by researchers skeptical about the big bang theory, the most formidable among whom was the British astrophysicist Fred Hoyle. A born outsider who had by sheer intellectual energy made his way from the gray textile valleys of the north of England to the high table at Cambridge, Hoyle was individualistic to the point of iconoclasm, and as combative as if he had earned his knighthood on horseback. He lectured charismatically, in a working-class accent that seemed if anything to deepen as his scholarly credentials accumulated, and he was equally effective with the written word, publishing incisive technical papers, engrossing popularizations of science, and sprightly science fiction yarns with a seemingly effortless facility. Fearful was his scorn, and withering were his critiques of the big bang theory.
Hoyle damned the theory as epistemologically sterile, in that it seemed to place an inviolable, temporal limitation on scientific inquiry: The big bang was a wall of fire, past which science at the time did not know how to probe. Hoyle found it “highly objectionable that the laws of physics should lead us to a situation in which we are forbidden to calculate what happened before a certain moment in time.”
10
He poked fun at the theory’s creationist overtones: Had it not been proposed by a priest, Lemaître, and had not Pope Pius XII, at the opening of a meeting of the Pontifical Academy of Sciences on November 22, 1951, declared that it accorded with the Catholic concept of creation (an endorsement that, Gamow joked, demonstrated its “unquestionable truth”)?
11
Empirically, Hoyle was unsparing in calling attention to the big bang theory’s most telling liability, the time-scale problem. Owing to a number of errors, chief among them an inadequate understanding of the absolute magnitude of the Cepheid variable stars employed as intergalactic distance indicators, Hubble and Humason had severely underestimated the dimensions of the expanding universe—and, therefore, its age as well. Hubble’s original statement of the expansion law had been that H
0
, the expansion parameter, equaled 550 kilometers per second per megaparsec—meaning that for every megaparsec (or 3.26 million light-years) that one looks out into space, one finds galaxies moving apart at an additional 550 kps.
The trouble was that this value for H
0
yielded an elapsed time since the big bang of only about two billion years. This was smaller than the age of the sun and the earth. Since the universe cannot be younger than the stars and planets it contains, obviously something was wrong.
In Hoyle’s view, what was wrong was the big bang concept itself. As an alternative, he and two colleagues, Herman Bondi and Thomas Gold, promulgated in 1948 what they called the steady state model. According to their theory, the universe was infinitely old and generally unchanging: There had been no creation event, no high-density infancy from- which the universe had evolved.
*
The steady state theory was not destined to prosper; it lost its raison d’être once the errors in Hubble’s distance figures were repaired, and it predicted that some galaxies ought to be very much older than others, of which no evidence has ever been found. But it had the salubrious effect of concentrating its advocates’ attention on the question of where the heavier elements had come from. The steady staters could scarcely imagine, as Gamow did, that the elements had been synthesized in the big bang, since they denied that there had ever
been
a big bang. Consequently they were obliged to find another furnace in which to cook up such wonderfully complex atoms as those of iron, aluminum, and tin. The obvious candidate was the stars.
Hoyle, who possessed a command of nuclear physics unsurpassed among the astronomers of his generation, had begun working on the question of stellar fusion reactions in the mid-1940s. He had published little, however, owing to a running battle with “referees,” anonymous colleagues who read papers and vet them for accuracy, whose adversity to Hoyle’s more innovative notions prompted him to stop submitting his work to the journals. Hoyle paid a price for his rebelliousness, though, when in 1951, while he stood stubbornly in the wings, Ernst Öpik and Edwin Salpeter worked out the synthesis
in stars of atoms up through beryllium to carbon. Rankling at the missed opportunity, Hoyle then broke his silence, and in a 1954 paper demonstrated how red giant stars could build carbon into oxygen-16.
Ahead still lay the seemingly insurmountable obstacle of iron. Iron is the most stable of all the elements; to fuse iron nuclei into the nuclei of a heavier element consumes energy rather than releasing it; how, then, could stars fuse iron and still shine? Hoyle thought that Supernovae might do the job—that the extraordinary heat of an exploding star might serve to forge the elements heavier than iron, if that of an ordinary star could not. But this he could not yet prove.
Then, in 1956, fresh impetus was lent to the question of stellar element production when the American astronomer Paul Merrill identified the telltale lines of technetium-99 in the spectra of S stars. Technetium-99 is heavier than iron. It is also an unstable element, with a half-life of only two hundred thousand years. Had the technetium atoms that Merrill detected originated billions of years ago in the big bang, they would since have decayed and there would be too few of them left to show up today in S stars or anywhere else. Yet there they were. Clearly the stars knew how to build elements beyond iron, even if the astrophysicists didn’t.
Spurred on by Merrill’s discovery, Hoyle renewed his investigations into stellar nucleogenesis. It was a task he took very seriously; as a boy, hiding atop a stone wall while playing hide-and-seek one night, he had looked up at the stars and resolved to find out what they were, and the adult astrophysicist never forgot his childhood pledge. Visiting the California Institute of Technology, Hoyle found himself in the company of Willy Fowler, a resident faculty member with an encyclopedic knowledge of nuclear physics, and Geoffrey and Margaret Burbidge, a talented husband and wife team who, like Hoyle, were English big bang skeptics.
A break came when Geoffrey Burbidge, scrutinizing recently declassified data from a Bikini Atoll bomb test, noticed that the half-life of one of the radioactive elements produced by the explosion, californium-254, was fifty-five days. This rang a bell: Fifty-five days was just the period that a supernova that Walter Baade studied had taken to fade away. Californium is one of the heaviest of all elements; if it were created in the intense heat of exploding stars, then surely the elements between iron and californium—
which comprise,- after all, most of the periodic table—could have formed there, too. But how?
Happily, nature had provided a Rosetta stone against which Hoyle and his collaborators could test their ideas, in the form of the cosmic abundance curve. This was a plot of the weight of the various atoms—some twelve hundred species of nuclei, when the known isotopes were taken into account—against their relative profusion in the universe, as determined by studying the rocks of the earth, meteorites that have fallen to earth from space, and the spectra of the sun and stars. Physicists working on the Manhattan Project and the hydrogen bomb tests that followed had grown accustomed to deciphering the chain reactions involved by studying the relative abundances of various isotopes found in the debris left behind by the explosion. The cosmic element abundance curve was, in a sense, just another such table writ large; Gamow called it “the oldest document pertaining to the history of our universe.”
12
But where for Gamow that history was principally the story of the big bang, for Hoyle and his colleagues the important thing was what had gone on since, inside a billion trillion stars. “The problem of element synthesis,” they would write, “is closely allied to the problem of stellar evolution.”
13
The differences in abundances are great—there are, for instance, two million atoms of nickel for every four atoms of silver and fifty of tungsten in the Milky Way galaxy—and the abundance curve consequently traced out a series of jagged peaks more rugged than the ridgeline of the Andes. The highest peaks were claimed by hydrogen and helium, the atoms created in the big bang—more than 96 percent of the visible matter in the universe is composed of hydrogen or helium—and there were smaller but still distinct peaks for carbon, oxygen, iron, and lead. The pronounced definition of the curve put welcome constraints on any theory of element synthesis in stars: All one had to do (though this was quite a lot) was to identify the processes by which stars had come preferentially to make some elements in far greater quantities than others. Here the genealogy of the atoms was inscribed, as in some as yet untranslated hieroglyph: “The history of matter,” wrote Hoyle, Fowler, and the Burbidges, “… is hidden in the abundance distribution of the elements.”
14
Their work culminated in 1957 in an epochal paper, 103 pages long, that showed how fusion processes operating in addition to
Bethe’s proton-proton reaction and carbon cycle could build the atoms of the heavy elements—the “metals,” which in astrophysical parlance means everything heavier than helium. The tentpole of the paper was the arrow of time: The evolution of atoms, it revealed, is bound up in the evolution of stars, and the mix of elements found in the universe today is largely the result of what stars did in the past. At first, a star is powered by “hydrogen burning,” the fusing of hydrogen nuclei to build helium. This is the proton-proton reaction discerned by Bethe, and it can go on for a long time, from about a million years for a furiously burning giant star to ten billion years or so for a more tepid star like the sun. “But,” as Hoyle, Fowler, and the Burbidges noted, “no nuclear fuel can last indefinitely.”
15
Eventually the supply of hydrogen runs low and the star’s core contracts. The contraction heats the core, and in the hotter environment helium burning can begin. The fusion of helium nuclei forms atoms of carbon, oxygen, and neon—but not lithium, beryllium,
or boron, which explains why the former elements show up as peaks on the cosmic element abundance curve and the latter as valleys. When this process falters, the core contracts and heats further, fusing helium nuclei with those of neon to build magnesium, silicon, sulphur, and calcium. Now the old picture of a split-personality star could be refined into multiple personalities: A highly evolved star sorts itself into layers, like an onion, its gaseous iron core surrounded by concentric shells where silicon, oxygen, neon, carbon, helium, and, in the outermost shell, hydrogen are being burned. And so it goes, through previously undiscerned displays of the virtuosity of stellar alchemy.
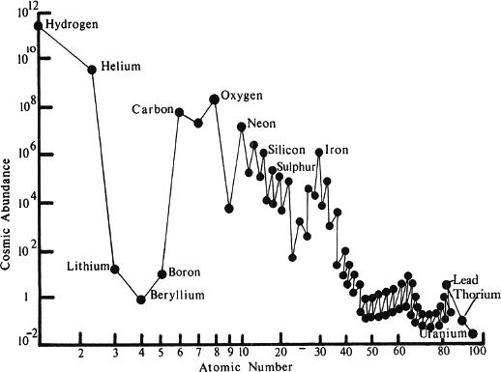
The cosmic element abundance curve depicts the relative numbers of various sorts of atoms found in the universe at large. It serves as a constraint on theories of how the elements formed. (After Taube, 1982.)
Iron spells death, and death deliverance. The iron core grows like a cancer in the heart of the star, damping nuclear reactions in all that it touches, until the star becomes fatally imbalanced and falls victim to a general collapse. If the mass of the core is a tenth to two or three times that of the sun—here we draw on research by Gamow, Baade, Robert Oppenheimer, Fritz Zwicky, and others—the core rapidly crystallizes into a steely sphere, a “neutron star.” Smooth as a ball bearing and smaller than a city but as massive as the sun, a neutron star spins rapidly on its axis and emits pulses of radio energy as it spins, creating a beacon of the sort that betrayed the locations of Tycho’s and Kepler’s Supernovae. It resembles nothing so much as a giant atomic nucleus—as if the real business of the star, the conjuring of nuclei, was now at last monumentalized as a colossal nuclear tombstone.
The bright side, literally so, is that the explosion of the star generates sufficient energy to synthesize an enormous variety of atoms heavier than iron. When the iron core collapses it emits a single great clang, and this final ringing of the gong sends a sound wave climbing upward through the inrushing gas from the envelope of starstuff left behind. As the sonic wave rushing outward meets the waves of gas falling in, the result is a shock stronger than any other in the known universe. In a moment, tons of gold and silver, mercury, iron and lead, iodine and tin and copper are forged in the fiery collision zone. The detonation blows the outer layers of the star into interstellar space, and the cloud with its freight of valuable cargo expands, marching out over the course of aeons to become entangled with the surrounding interstellar clouds. When latter-day stars condense from these clouds, their planets inherit the star-forged elements. The earth was one such planet, and such
is the ancestry of the bronze shields and steel swords with which men have fought, and the gold and silver they fought over, and the iron nails that Captain Cook’s men traded for the affections of the Tahitians.