The Dancing Wu Li Masters (9 page)
Read The Dancing Wu Li Masters Online
Authors: Gary Zukav

Planck is not only the father of quantum mechanics, he also is the discoverer of Planck’s constant. Planck’s constant is a certain number which never changes.
*
It is used to calculate the size of the energy packets (quanta) of each light frequency (color). (The energy in each light quantum of a particular color is the frequency of the light multiplied by Planck’s constant.)
All of the energy packets of each color have the same amount of energy. All of the energy packets of red light, for example, are the same size. All of the energy packets of green light are the same size. All of the energy packets of violet light are the same size. The energy packets of violet light, however, are larger than the energy packets of green light, and the energy packets of green light are larger than the energy packets of red light.
In other words, Planck discovered that energy is absorbed and emitted in little chunks and that the size of the chunks of a low-frequency light, like red, is smaller than the size of the chunks of a high-frequency light, like violet. This explains why hot objects radiate energy as they do.
When a black body is put over low heat, the first color it glows is red because the energy packets of red light are the smallest energy packets in the visible light spectrum. As the heat is increased, more energy is available to shake loose bigger energy packets. The bigger energy packets make the higher-frequency colors, such as blue and violet.
Why does the glow of hot metal seem to increase steadily in brightness as the temperature increases? Because the tiny “steps” upward and downward in brightness are so incredibly small that our eyes cannot discern them. Therefore, on the large scale, or macroscopic level, this aspect of nature is not evident. In the subatomic realm, however, it is the dominant characteristic of nature.
If this discussion of emission and absorption of energy packets reminds you of Niels Bohr, you are right. However, Bohr was not to arrive at his theory of specific electron orbits for another thirteen years. By that time physicists had discarded the plum-with-jiggling-electrons model of the atom in favor of the planetary model, in which electrons revolve around a nucleus.
*
Between Planck’s discovery of the quantum (1900) and Bohr’s analysis of the hydrogen spectrum (1913), a brilliant physicist burst upon the scene with a force seldom exerted by an individual. His name was Albert Einstein. In one year (1905), at twenty-six, Einstein published five significant papers. Three of them were pivotal in the devel
opment of physics, and, to a large extent, in the development of the West. The first of these three papers described the quantum nature of light. It won him a Nobel Prize in 1921. The second paper described molecular motion. The third paper set forth the special theory of relativity, which we will study later.
*
Einstein’s theory of light was that it is composed of tiny particles. A beam of light, said Einstein, is analogous to a stream of bullets. Each bullet is called a photon. This is similar to what Planck proposed, but actually it is a leap beyond. Planck discovered that energy is absorbed and emitted in packets. He described the
processes
of energy absorption and emission. Einstein theorized that
energy itself
is quantized.
To prove his theory, Einstein referred to a phenomenon called the photoelectric effect. When light hits (impinges on) the surface of a metal, it jars electrons loose from the atoms in the metal and sends them flying off. With appropriate equipment, we can count these electrons and measure how fast they are traveling.
Einstein’s theory of the photoelectric effect was that each time one of the bullets, or photons, hits an electron, it knocks it away just as one billiard ball hitting another billiard ball knocks it away.
Einstein based his revolutionary theory on the experimental work of Philippe Lenard (who won the Nobel Prize in 1905). Lenard showed that the flow of electrons in the photoelectric effect begins immediately when the impinging light strikes the target metal. Turn on the light and out come the electrons. According to the wave theory of light, the electrons in a metal only start to jiggle when they are struck by light waves. They do not come out of the metal until they are moving fast enough. This takes several oscillations, like pumping a child’s swing higher and higher until it goes around the bar. In short, the wave theory of light predicts a delayed emission of electrons. Lenard’s experiments showed a prompt emission of electrons.
This prompt emission of electrons in the photoelectric effect is explained by Einstein’s particle theory of light. Every time a particle of light, a photon, strikes an electron, it immediately knocks it out of its atom.
Lenard also discovered that reducing the intensity of the impinging light beam (making it dimmer) did not reduce the velocity of the rebounding electrons, but it did reduce the number of the rebounding electrons. He found that the velocity of the rebounding electrons could be altered, however, by changing the
color
of the impinging light.
This also was explained by Einstein’s new theory. According to Einstein’s theory, each photon of a given color, like green, for instance, has a certain amount of energy. Reducing the intensity of a beam of green light only reduces the number of photons in the beam. Each remaining photon, however, still has the same amount of energy as any other photon of green light. Therefore, when any photon of green light strikes an electron, it knocks it away with a certain amount of energy which is characteristic of green-light photons.
Max Planck described Einstein’s theory this way:
…The photons (the “drops” of energy) do not grow smaller as the energy of the ray grows less; what happens is that their magnitude remains unchanged and they follow each other at greater intervals.
2
Einstein’s theory also substantiated Planck’s revolutionary discovery. High-frequency light, like violet, is made of higher-energy photons than low-frequency light, like red. Therefore, when violet light, which is made of high-energy photons, strikes an electron, it causes the electron to rebound with a high velocity. When red light, which is made of low-energy photons, strikes an electron, it causes the electron to rebound at a low velocity. In either case, increasing or decreasing the intensity of the light increases or decreases the number of rebounding electrons, but only by changing the color of the impinging light can we change their velocity.
In short, Einstein demonstrated, using the photoelectric effect,
that light is made of particles, or photons, and that the photons of high-frequency light have more energy than the photons of low-frequency light. This was a momentous achievement. The only problem was that one hundred and two years earlier an Englishman named Thomas Young had shown that light is made of waves, and no one, including Einstein, was able to disprove him.
Now we come to the matter (no pun) of waves. A particle is something that is contained in one place. A wave is something that is spread out. Here are some types of waves.
We are concerned only with the last type of wave. Here is a more detailed picture of it.
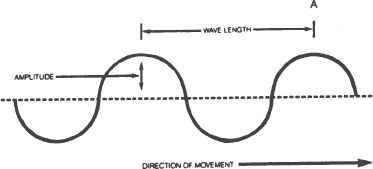
A
wavelength
is the distance between one crest of a wave and the next. The longest radio waves are over six miles long. X-rays, on the other hand, are only about one billionth of a centimeter long. Visible light has wavelengths in the neighborhood of four to eight one hundred thousandths of a centimeter.
The
amplitude
of a wave is the height of the wave crest above the dotted line. Here are three waves with different amplitudes. The one in the middle has the largest amplitude.
The
frequency
of the wave tells us how many crests pass a given point (like Point A in the drawing) each second. If the wave is moving in the direction of the arrow and a crest passes point A each second, the frequency of the wave is one cycle per second. If ten and one half crests pass point A every second, the frequency of the wave is 10.5 cycles per second. If ten thousand crests pass the same point every second, the frequency of the wave is 10,000 cycles per second, and so on.
The velocity of the wave can be determined by multiplying the wavelength by the frequency. For example, if the wavelength of a wave is two feet and the frequency of the wave is one cycle per second, the wave is moving one wavelength (two feet) every second. Therefore, its velocity is two feet per second. If the wavelength is two feet and the frequency is three cycles per second, the velocity of the wave is six
feet per second because the wave moves three wavelengths forward every second.
There is nothing complicated about this. We can determine how fast a man is running if we know the length of his stride and how many of them he takes in a second. By multiplying them together we get how far the man runs in a second. If his stride is three feet and he takes two strides per second, then he runs six feet per second (about four miles per hour). We do the same things with waves, except that we use wavelengths instead of strides.
Although the velocity of a light wave
can
be determined by multiplying its wavelength by its frequency, it is not necessary. Physicists have discovered that the velocity of light in empty space is
always
186,000 miles per second. This applies to all electromagnetic waves, including light. Therefore, all light waves (blue ones, green ones, red ones, etc.) have the same velocity as radio waves, x-rays, and all the other forms of electromagnetic radiation. The speed of light is a constant. It is represented by the letter “c.”
The constant “c” is (approximately) 186,000 miles per second and it never varies (which is what makes it a “constant”). It does not matter whether light is going up or down, has a high frequency or a low frequency, a large wavelength or a small wavelength, is coming toward us or going away from us: Its velocity is always 186,000 miles per second. This fact led Albert Einstein to the theory of special relativity, as we shall see later.
It also permits us to know both the frequency and the wavelength of light if we know either one of them. This is because the product of the two is always 186,000 miles per second in empty space. The larger one of them is, the smaller the other must be. For example, if we know that by multiplying two numbers together we get 12 for an answer, and if we know that one of the numbers is 6, then we also know that the other number
must
be 2. If we know that one of the numbers is 3, then we know that the other number
must
be 4.
Similarly, the higher the frequency of a light wave, the shorter its wavelength must be; the lower the frequency of a light wave, the
longer its wavelength must be. In other words, high-frequency light has a short wavelength and low-frequency light has a long wavelength.
Now we return to Planck’s discovery. Planck discovered that the energy of a light quantum increases with frequency. The higher the frequency, the higher the energy. Energy is proportional to frequency, and Planck’s constant is the “constant of proportionality” between them. This simple relation between frequency and energy is important. It is central to quantum mechanics. The higher the frequency, the higher the energy; the lower the frequency, the lower the energy.