Murder and Mayhem (35 page)
Authors: D P Lyle

Q: In my story an elderly man with early-stage Alzheimer's is found floating in a bay, but he was actually drowned earlier in a backyard swimming pool. Would chlorine from the pool show up in the man's system during autopsy? Because the victim is initially presumed to have drowned in the bay, would the medical examiner expect to find debris, such as small bits of vegetation or the like, in his lungs? What would the M.E. be looking for in a drowning situation like this?
A: In drownings the M.E. can determine if it was freshwater or salt water and should be able to determine if the water contained
chlorine. This freshwater versus salt water distinction was used in the movie
Chinatown.
To understand the differences between freshwater and saltwater drownings, let's first take up the issue of osmosis. Osmosis is the passage of a liquid through a semipermeable membrane driven by a concentration gradient. Simple, huh? Let me explain. We use the term "tonicity" to describe the concentration of electrolytes (sodium, potassium, chloride, and so forth) in a liquid. The major electrolyte in the human body and in the blood is salt or sodium chloride (NaCl). "Isotonic" means the liquid has the same tonicity or NaCl concentration as blood. If the tonicity is less, as in fresh or pool water, it is termed hypotonic ("hypo" means lower or less). If the tonicity is higher, as in salt water, which contains a higher concentration of salt than blood, it is called hypertonic ("hyper" means above or more).
For our discussion a semipermeable membrane is a barrier that allows water to pass through but not other molecules such as sodium chloride. Water moves across this barrier from the hypotonic (lower concentration) liquid toward the hypertonic (higher concentration) liquid (Figure 19a). This movement continues until the tonicity on each side of the membrane is the same. In reality the water molecules continue to move back and forth, but once the tonicity is equal, the movement of water in each direction is equal. Think of it as the relatively hypertonic liquid acting like a sponge that "pulls" water toward it. Once things balance on each side of the membrane, this sponge effect is lost.
The tissues of the lungs are semipermeable membranes designed to allow oxygen and carbon dioxide free movement back and forth. The blood that bathes the lung tissue is isotonic.
In a freshwater drowning (Figure 19b), hypotonic fluid is introduced into the lungs. This causes water to move from the water-filled air sacs of the lungs into the bloodstream. This occurs because the isotonic blood is actually hypertonic relative to the hypotonic freshwater. This movement of water dilutes the blood,
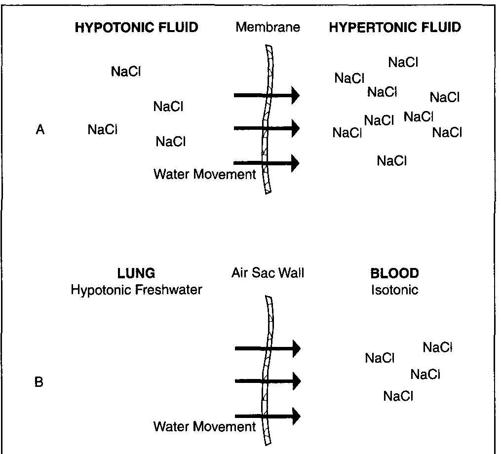
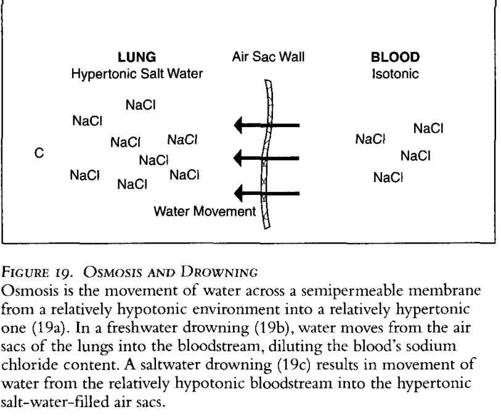
making it hypotonic relative to what it should be. In a saltwater drowning (Figure 19c), the opposite occurs. Salt water is hypertonic relative to blood, so water moves out of the bloodstream and into the air sacs of the lungs.
The M.E. would examine the lungs and the blood of the victim. In a freshwater drowning he would expect to see relatively dry air sacs (the water has moved into the bloodstream) and diluted or hypotonic blood. In a saltwater drowning he would expect to see wet air sacs (the water moves from the blood toward the salt-water-filled lung tissue) and concentrated or hypertonic blood. If he didn't find what was expected, he might conclude the body had been moved. It is important to note that this process of water movement doesn't happen instantly, so if the victim is pulled from the water quickly, little change may be detectable on these exams.
In your scenario the M.E. could find evidence for freshwater drowning and could find the lungs contained some residual chlorinated water. He would deduce that the victim had drowned in a swimming pool and not in a saltwater bay.
Yes, the victim's mouth, throat, and even his lungs could contain debris, vegetation, algae, and even small aquatic creatures, which would be those found in the area of drowning. There would be a mismatch if the body was moved to a different location after death. This was used in the film
Silence of the Lambs
when the larvae found in the throat of one victim was that of a rare moth that wasn't indigenous to the United States. The same can be said for vegetation. Of course, in a swimming pool the victim would probably not aspirate any vegetation or debris. If he drowned in a pond or lake, then freshwater vegetation or bugs rather than chlorine would be the findings that cause the M.E. concern. Here the M.E. would state that the victim was moved because the drowning was freshwater and the vegetation and bugs were freshwater life forms; thus, the victim could not have drowned in the saltwater bay.
Will an Autopsy Determine If Chlorine Is Present in the Lungs?
Q: I know the M.E. can determine whether a drowning is freshwater or salt water, but can chlorine be detected in a drowning victim and lead to the conclusion that the drowning occurred in a pool rather than a bathtub?
A: Yes. Samples of the lung tissue and any liquid that the lungs contain can be tested for chlorine. Some labs have to send the samples away to a more sophisticated lab for analysis, but the chlorine can be detected in most cases.
Do Skin and Nail Changes Occur with Some Poisons?
Q: I've read that some poisons can be determined by examining the skin and fingernails of the victim. Is this true? Do you have any examples of which poisons can be detected this way?
A: Yes. Many poisons cause changes in the skin, hair, mucous membranes of the mouth, and nails.
Lead:
Chronic lead poisoning (plumbism) has been around for many centuries and may have led the decline of the Roman and Greek empires. Today it usually comes from exposure to lead-containing paints, gasoline, and plumbing pipes, and cooking or eating from ceramic containers that are finished with lead-containing glazes. Plumbism is associated with anemia, headache, abdominal pain, joint pain, fatigue, memory problems, neuropathies (weakness and/or numbness in an extremity), and a blue-black line at the junction of the teeth with the gums, called a "lead line."
Mercury:
In children mercury poisoning can cause a syndrome known as acrodynia or pink disease. It is characterized by flushing, itching, swelling, excessive salivation and sweating, weakness, red, irregular skin rashes, and scaling of the skin on the palms and feet.
Arsenic:
Chronic exposure to arsenic can cause hyperkeratosis and hyperpigmentation (thickening and darkening) of the skin, exfoliative dermatitis (flaking and sloughing of the skin), and transverse white lines in the fingernails, called Mee's Lines. Arsenic can be detected in the hair of victims of chronic poisoning.
Cyanide:
Cyanide is a metabolic poison in that it blocks cytochrome oxidase, an enzyme found in the mitochondria of the cells. The mitochondria are responsible for cellular energy production and oxygen utilization. Blocking cytochrome oxidase prevents the cells from using oxygen, resulting in their death.
Cyanide forms cyanmethhemoglobin through a complex interaction with the hemoglobin found in the red blood cells, which lends a bright cherry red color to the blood. Because of this it can be confused with carbon monoxide poisoning (see below).
Hypostasis (lividity) is the postmortem settling of blood in dependent areas due to gravity. Typically, this is blue-gray or purple in color. With cyanide poisoning the cyanmethhemoglobin imparts a reddish hue to the settling blood so that dependent lividity in this situation takes on a brick red or dark pink color.
Carbon monoxide:
Carbon monoxide combines with the hemoglobin in the red blood cells to form carboxyhemoglobin, which gives the blood and tissues a cherry red hue.
Is There a Poison That Can't Be Detected or That Can Be Masked by Venom?
Q: For my story I need to know if there is a poison that either will not leave a forensics fingerprint or that can be covered by either scorpion venom or that of a rattlesnake.
A: Your best bet would be succinylcholine. It is an injectable muscular paralytic that paralyzes all muscles. The victim is awake and alert but can't move, speak, bat an eye, or breathe. Death is in about three or four minutes. The drug is quickly broken down in the body, so the M.E. is unlikely to find the drug even if he tests for it—with one exception.
If the injection site is visible, he could excise the tissue in the area and test it for the drug's breakdown products. As the drug is destroyed by the body's enzymes, it is converted to other compounds, and these compounds are called "breakdown products." Remnants of these substances would be left behind in the tissues near the point of injection. The famous case of Carl Coppolino's murder of his wife was solved using this technique. Coppolino was an anesthetist, so he had access to the drug. His wife's body was exhumed and the injection site located. Tissue testing gave the results needed to convict him.
If the M.E. found venom, a bite or sting site, and skin and blood changes that went along with the venom, he would assume that the cause of death was due to the venom. Looking for an injection site and testing for succinylcholine breakdown products would probably not be considered.
One caveat: The venom must be given while the victim is alive. Its local tissue destruction and its destructive effects on blood cells ceases at death when the circulation and all the body's metabolic processes stop. The M.E. would need to see the effects of the venom to conclude that it was the proximate cause of death. The victim could be paralyzed, maybe partially with a series of small doses of succinylcholine, and then exposed to the snake or scorpion. The victim would indeed die from the venom, but his death wouldn't be "accidental."
Succinylcholine can be found in hospital pharmacies, emergency rooms, and operating suites, so it could be stolen. Or it could be ordered from a pharmaceutical supplier.
Do Postmortem Wounds Bleed?
Q: If a person is murdered with a poison and then within a half hour a dagger is stuck into his throat to make it look as if the dagger was the cause of death, would the person bleed much?
A: I assume you mean the person was stabbed after death from the poison. In that case he would not bleed since the blood in the body clots fairly quickly once the action of the heart has ceased and the blood stagnates. The M.E. would be able to determine that the wound occurred after death in most cases.
If, on the other hand, the victim was stabbed when he was incapacitated by the poison but still alive, he would bleed, and the fact that he was also poisoned would have to await the M.E.'s toxico-logical studies.
What Do "Mood" Cosmetics Look Like on a Corpse?
Q: What would happen to "mood" cosmetics (lipstick and nail polish) that change color on live bodies with warm and cool temperatures after that person becomes a corpse?
A: Cosmetics are topical; that is, they sit on the surface of the lips and nails and do not interact with the tissues of the body. Thus, the product wouldn't know if the wearer was dead or alive. If the product actually interacted with the body's tissues, it would be designated a pharmaceutical agent, would come under FDA scrutiny, and wouldn't be classified as a cosmetic.
These "mood" products, predominantly lipstick and nail polish, react to heat. They change color over a specified range depending on the temperature. They are marketed to teenage girls because they "change colors with your body heat and mood." The color changes depend on the manufacturer and the particular product, but they tend to brighten as body temperature increases. I guess that way people can tell whether the wearer is "cool" or "hot."