Life on a Young Planet (27 page)
Read Life on a Young Planet Online
Authors: Andrew H. Knoll

In addition to microfossils, Roper shales contain molecular fossils, including steranes that provide complementary evidence for eukaryotic life. Even macroscopic fossils of likely eukaryotic origin occur in middle Proterozoic rocks. Observant paleontologists in Australia, China, India, and the United States have uncovered helical compressions an inch or so across in mid-Proterozoic siltstones as well as short strings of beads 1–3 millimeters in diameter preserved as impressions on the surfaces of
sandstone beds. These fossils are hard to classify; conceivably, they record extinct lineages only loosely related to modern eukaryotes.
Collectively, then, paleontological discoveries indicate that the late Proterozoic ascendance of algae and protozoans was not the starting gun for eukaryotic life. Nor is the documented increase in eukaryotic diversity simply an artifact of rock preservation or sampling.
Something
—some biological innovation or environmental shift—must have happened to spur eukaryotic diversification toward the end of the Proterozoic Eon.
If the rise of eukaryotes to ecological and taxonomic prominence came long after the origin of eukaryotic cells, we have to think about triggers for diversification, either biological or physical. What about sex? The undeniable (and, perhaps, not wholly scientific) attraction of this proposition rests on a simple observation and a bit of arithmetic. The observation is taxonomic: about 4,000 bacteria have been given species names; in contrast at least 100,000 protozoans and algae, another 100,000 fungi, some 300,000 land plants, and more than a million animals have been described. The math comes in to bolster the argument that sex promotes diversity by enabling eukaryotes to generate the raw material for evolution—genetic variation within a population—differentially well. Bill Schopf is especially fond of this idea, which he illustrates by means of a simple thought experiment. In bacteria that reproduce by binary cell division, ten mutations that arise in a population will result in a maximum of eleven different combinations of genes. But, let those same ten mutations arise in a sexually reproducing population of eukaryotes, and the possible genetic combinations run into the thousands. No wonder eukaryotes are so diverse. The argument is comfortably simple, it makes intuitive sense, and it relies on natural history of the kind that “every schoolboy knows.” In short, it is just the sort of proposition that graduate students love to dismantle.
Let’s start with the statistics on species diversity. Named bacterial species have one trait in common—they can be grown in the laboratory. Using new molecular techniques, however, microbial ecologists have found that culturable organisms represent as little as 1 percent of the bacterial diversity present in natural environments. Thus, the true diversity of bacteria may rival that of protozoans and algae.
We also have to poke a bit at the issue of genetic variability, because it makes the important assumption that bacteria have no means of combining genes from two individuals. As noted already in
chapter 2
, this assumption is spectacularly incorrect. The well-known intestine dweller
E. coli
, for example, practices a form of sex in which two cells become tethered by a tiny tube, allowing genetic material to pass from one to the other. Other bacteria take up small lengths of DNA released to the environment by dying cells. Still others incorporate genetic material transported by viruses. In fact, bacteria exchange genetic material all the time, and they do it not only between two individuals in the same population but between distinct species and even between kingdoms. If sex is defined as the exchange of genetic material between individuals, bacteria are decidedly sexy. Prokaryotes do not suffer from a poverty of genetic variation.
Thus, if we want to entertain the notion of sex as a trigger for eukaryotic diversification, we have to posit that early eukaryotes lacked both sex and the mechanisms for genetic exchange found in bacteria. At present, however, we don’t know this. We really don’t know when sexual reproduction entered the eukaryotic life cycle, if in fact it wasn’t already present in the last common ancestor of all living protists. Perhaps we should approach the problem differently.
The “just add sex” hypothesis assumes that diversity is somehow constrained by the rate at which genetic variation can be generated, but diversity depends as much if not more on biological function and ecology. Prokaryotic diversity reflects the remarkable ability of bacteria and archaea to exploit specific nutrient sources and energy gradients. In contrast, eukaryotes diversified by approaching the world in new ways. As discussed in the preceding chapter, the eukaryotic cytoskeleton and membrane system enables nucleated cells to do something that bacteria can’t do at all—swallow particles, including other cells. Thus, as evidenced by the vase-shaped microfossils in the Grand Canyon, eukaryotes introduced grazing and predation into microbial ecosystems. Doggerel by Jonathan Swift captures the consequences nicely:
So, naturalists observe, a flea
Has smaller fleas that on him prey;
And these have smaller still to bite ‘em;
And so proceed ad infinitum.
By expanding ecosystem complexity, eukaryotic cells erected a new scaffolding for diversity.
Eukaryotic organisms do something else that has largely eluded prokaryotes. Plants, animals, fungi, and seaweeds develop via a complex pattern of cell division and differentiation choreographed by molecular signals that pass from cell to cell, switching specific genes on or off as they go. This beautifully coordinated regulatory system may have its origins in single-celled organisms that changed size or shape as they passed through the cell cycle, but it eventually made possible the evolution of complex multicellularity, and in so doing fueled a further expansion of eukaryotic diversity. Ninety-five percent or more of extant eukaryotic species are multicellular.
At the end of the day, it isn’t necessary (or, perhaps, wise) to focus too narrowly on any one trait as key to eukaryotic diversification. Sex, cytoskeletons, genetic regulation, and, doubtless, other characters interacted to produce the plethora of eukaryotic forms seen today. No one knows when the modern eukaryotic “tool kit” was assembled, but the small fossils in Roper shales suggest that it was in place long before the diversification recorded in late Proterozoic rocks.
This brings us to the remaining explanation for late Proterozoic diversification—changing environments. Can we envision environmental changes that might have improved the odds of eukaryotic success in a prokaryotic world? If we can, do the rocks provide evidence that the required changes actually took place in concert with eukaryotic diversification? Increasingly, the answer to both questions appears to be yes.
In
chapter 6
, we discussed evidence for an increase in atmospheric (and surface ocean) oxygen 2.2–2.4 billion years ago. When I was a student in the 1970s, this event was generally accepted as the transition between Earth’s two great environmental states: the Archean to earliest Proterozoic, when O
2
was scarce, and the past 2 billion years, during which air and ocean have both been bathed in oxygen. But, as noted in
chapter 6
, Odense University’s Don Canfield has proposed that what the early Proterozoic oxygen revolution ushered in was not the modern world, but rather an alien intermediate marked by moderate oxygen in the atmosphere and surface sea and hydrogen sulfide in deep waters (
figure 9.6
).
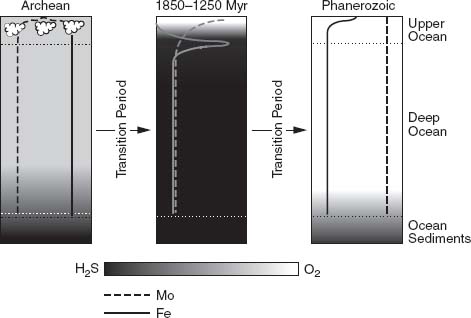
Figure 9.6.
Triptych illustrating the three phases of ocean evolution. Early oceans contained little oxygen, but relatively abundant iron. Modern oceans contain abundant oxygen and little iron. In between, during a long-lived state that may have lasted from 1.8 billion years ago until near the end of the Proterozoic Eon, the oceans are thought to have had moderate oxygen in surface waters, but hydrogen sulfide at depth. In such an ocean, biologically important trace elements such as iron and molybdenum (concentrations, higher to the right, illustrated by vertical lines) may have been in seriously short supply. (Reprinted with permission from A. D. Anbar and A. H. Knoll, 2002. Proterozoic ocean chemistry and evolution: a bioinorganic bridge?
Science
297: 1137–1142. Copyright 2002 American Association for the Advancement of Science)
Those fossiliferous rocks from northern Australia provide a good test of Don’s hypothesis (maximizing my scientific return per tick bite). Putting our heads together, postdoctoral fellow Yanan Shen, Don, and I have been able to show that shales deposited in deeper parts of the Roper (and two older) basins preserve chemical signatures similar to those observed today in sediments at the bottom of the Black Sea. The Black Sea is well known to Earth scientists because its oxygen-rich surface layer blankets a much larger volume of water that fairly bristles with H
2
S. Independent work by Tim Lyons at the University of Missouri and my former student Linda Kah, now at the University of Tennessee, corroborates and expands this view of distinctive mid-Proterozoic oceans.
Why should this matter to evolving eukaryotes? Ariel Anbar, a talented geochemist at the University of Rochester, pointed out to me that in seawater like that envisioned for the mid-Proterozoic, the essential nutrient nitrogen would be relatively scarce.
5
That is okay for cyanobacteria because they can fix nitrogen and, furthermore, are remarkably adept at scavenging biologically useful forms of nitrogen from their surroundings. But the situation is different for photosynthetic eukaryotes. Algae thrive today where levels of nitrate (NO
3
−
, a biologically usable form of nitrogen that is readily available in modern seawater) exceed short-term requirements for growth, allowing cells to stockpile nutrients as a hedge against leaner times. In the mid-Proterozoic ocean, however, this would have been difficult, because nitrate can’t build to high levels when oxygen is limited and sulfide lurks beneath the surface ocean. Algae can’t fix nitrogen, and they compete poorly against cyanobacteria for the scarce nitrogen compounds that would have been present in mid-Proterozoic seawater. Moreover, to make use of such nitrate as might have been present, algae would have needed the metallic element molybdenum.
“Moly” is an essential ingredient in nitrate reductase, the enzyme that makes nitrate useful to organisms. Today, moly is widely available as a trace constituent of seawater, but in the alien world of the “Canfield” ocean, this element would have behaved somewhat differently. Then as now, moly would have been weathered from continental rocks and carried into the sea by oxygenated rivers. Unlike its distribution today, however, moly would have been common
only
in coastal waters where the rivers entered the sea. Farther from shore, surface waters would have mixed with subsurface water masses, causing molybdenum to be removed by reaction with hydrogen sulfide. Algae that washed into the open ocean would, thus, have faced the insult of molybdenum deprivation to go with the injury of nitrogen limitation.
All this suggests that 1.5 billion years ago, life might have been tough for eukaryotic algae. (Protozoans should have fared better, because they obtain the nitrogen they need from the cells they eat. But remember that
the paleontological record provides few recognizable glimpses of early protozoans—with the conspicuous exception of those vase-shaped fossils in Grand Canyon rocks, the fossils that document late Proterozoic eukaryotic expansion are largely algal.) Returning once more to rocks of the Roper Group, we can ask how presumed algae were distributed in this mid-Proterozoic seaway. Our prediction is that photosynthetic eukaryotes should have been most abundant and diverse along the ancient seacoast, where nitrate levels would have been highest and molybdenum most readily available. That, it turns out, is just what we see.
Indeed, it appears that, globally, eukaryotic algae first took root in coastal waters and only later spread across continental shelves. This ecological expansion is poorly documented, but in general it appears to have commenced about 1.2 billion years ago as nitrogen limitation in the “Canfield” ocean began to weaken. Granted new ecological opportunities, both seaweeds and plankton diversified—as we see in the fossil record. Protozoans like those buried deep within the Grand Canyon must have diversified, too, as heterotrophs learned to exploit the new
biological
environments created by the algae.
Thus, as our view of Proterozoic geology and paleontology strengthens, it once again implicates environmental history in the determination of evolutionary pattern. That doesn’t let biological innovation off the hook—new functional possibilities, especially those presented by multicellularity, must also have stoked the flame of eukaryotic diversification—but it requires us to seek evolutionary explanation in the
interaction
between genetic possibility and environmental opportunity.