Life on a Young Planet (22 page)
Read Life on a Young Planet Online
Authors: Andrew H. Knoll

Bacteria may have evolved by exchanging genes, but eukaryotes went them one better. Chloroplasts and mitochondria, the seats of energy metabolism in eukaryotic cells, arose by the lateral transfer of entire cells. Electron microscopy and molecular biology illuminate many aspects of eukaryotic cell evolution, but we still don’t understand how our own domain originated.
B
EYOND OUR
distantmost candle of fact lies a seductive darkness. Scientists are drawn to this blackness because we know it hides more candles, as yet unlit. We strike matches of hypothesis in the hope that a new wick will catch fire. Hypotheses seek to explain what we know, but more important, they make predictions about what we don’t know—about experiments not yet run, or fossils not yet discovered. For this reason, hypotheses provide built-in criteria for evaluation: do they help us to light the next candle or not?
Most hypotheses turn out to be wrong—some gloriously and others ignominiously so. This isn’t because scientists are dim or the exercise futile. It simply reflects the difficulty of fashioning a lasting explanation of nature. In fact, most hypotheses include useful ideas that survive to become part of the next model or scenario. Good hypotheses also spur new research and so provide value even when the research shows them to be flawed. Most of us develop hypotheses destined for modest success or failure, but on rare occasions an idea comes along that changes how we think about nature. Konstantin Sergeevich Merezhkovsky made one such proposal.
Merezhkovsky, professor of botany at Kazan University, hypothesized in 1905 that the cells of algae and plants are chimeras made up of two originally independent organisms united in obligatory and permanent partnership. Specifically, Merezhkovsky proposed that the chloroplast—the seat of photosynthesis in eukaryotic cells—originated as a cyanobacterium that was swallowed by a protozoan. In framing this hypothesis, he was attempting to explain an observation made years earlier by the German botanist A.F.W. Schimper. Pushing the limits of nineteenth-century microscopy, Schimper had observed that chloroplasts grow and divide independently of (although in synchrony with) the cells that surround them. Just as Pasteur had established that all life springs from life, Schimper showed that chloroplasts lost from cells cannot be generated anew—chloroplasts spring always and only from chloroplasts. Merezhkovsky was also familiar with research showing that corals and some other animals harbor symbiotic algae in their tissues. Insightfully, he combined these two observations to reach his remarkable conclusion: “Chlorophyll bodies grow, are nourished, synthesize proteins and carbohydrates, hand down their characteristics—all independent of the nucleus. In a word, they behave like independent organisms and should be examined as such. They are symbionts, not organs.”
Merezhkovsky’s hypothesis of endosymbiosis (two cells yoked in mutually beneficial partnership, with one cell nested inside the other) excited vigorous debate in its time, but eventually faded from view, undercut by neglect as much as by experimental refutation. Questions accumulated faster than answers—how did the symbiont become established inside the host’s cytoplasm? how did the symbiont fall under the genetic spell of the nucleus?—and in the absence of compelling explanations, biologists moved on to more tractable problems. By the early 1960s endosymbiotic theory drew mention in an American textbook only as “a bad penny that has been in circulation far too long.” In Soviet encyclopedias, Merezhkovsky was remembered for his contributions to systematic botany; elsewhere he was rarely remembered at all.
In the fall of 1972, I was an undergraduate looking for a term paper topic in botany. Sensing my need for direction, my professor steered me toward some then recent publications by Lynn Margulis, a young cell biologist with radical ideas. In a 1967 paper that I later learned was
rejected fifteen times before finding a home in the
Journal of Theoretical Biology
, she (as Lynn Sagan) reinvented the endosymbiotic hypothesis for eukaryotic cell origins. (Lynn wasn’t consciously resuscitating the theories of Merezhkovsky; in 1967 she had never heard of him.) Lynn proposed not only that chloroplasts had originated as endosymbiotic cyanobacteria, but also that mitochondria, the compartmentalized sites of respiration in eukaryotic cells, were descended from free-living, respiring bacteria.
In Darwin’s great vision, evolution is fundamentally a process of branching, of divergence—new forms and physiologies arise as the descendants of a common ancestor grow ever more different from one another. Lynn Margulis, however, argued for the emergence of evolutionary novelty as branches
fused.
In her view, each cell in my body reflects the union of two genetic lineages; the rose bush outside my window, blessed with chloroplasts as well as mitochondria, combines three distinct lines of descent. For me, the electric effect of Lynn’s paper was both immediate (I found my term paper topic) and lasting (early life, I decided, was the field for me).
Today, biologists accept the endosymbiotic origins of chloroplasts and mitochondria as fact, and Lynn Margulis owns the National Medal of Science. But why did she succeed where Merezhkovsky had failed? Simply put, biologists of the late twentieth century had tools at their disposal that earlier generations could not have imagined. With electron microscopy came the observation that chloroplasts and cyanobacteria share a common structural organization. Biochemistry further showed that cyanobacteria and chloroplasts are nearly identical in the molecular details of photosynthesis. Moreover, chloroplasts were found to respond to antibiotics as bacteria do, not like the nucleus and cytoplasm of the cells in which they occur. Perhaps most surprisingly, chloroplasts turned out to contain DNA, RNA, and ribosomes—the basic molecular machinery for cellular growth and replication.
In tandem, electron microscopic and biochemical research uncovered another striking feature of algal cells. Chloroplasts in red and green algae (and their descendants, the land plants) are surrounded by two membranes. The outer membrane is synthesized by the surrounding cytoplasm, following genetic instructions issued from the nucleus. In contrast, the inner membrane is made by the chloroplast itself. Moreover,
the outer chloroplast membrane is part of an extensive membrane system that includes the cell’s bounding membrane, the nuclear membrane, and an internal membrane system that permeates the cytoplasm. These membranes are in dynamic continuity, which means that while they may be distinct and unconnected at any one moment, they occasionally combine to form a complex and nearly continuous surface. The significance of this seemingly arcane detail is that the nucleus and cytoplasm lie within this membranous boundary, whereas the chloroplast and its inner membrane lie
outside
it (
figure 8.1
).
Lynn Margulis argued that these observations can all be explained by the endosymbiotic hypothesis, forcing biologists to reevaluate an idea long dismissed. The clinching evidence, however, came from molecular biology. As we saw in
chapter 2
, comparisons of nucleotide sequences in genes provide a powerful means of determining evolutionary relationships among organisms. Knowing this, we can construct an elegant test
of the endosymbiotic hypothesis. Merezhkosvky’s and Margulis’s proposal is fundamentally genealogical—an evolutionary merger uniting microbes from two distinct domains of life. If the hypothesis is correct, gene sequences from chloroplast DNA should be more similar to those of cyanobacteria than they are to genes in the nuclei of plant and algal cells. This turns out to be the case; in the Tree of Life, chloroplasts nestle among the cyanobacteria.
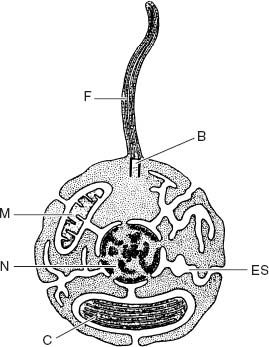
Figure 8.1.
The internal organization of the eukaryotic cell. Note that the membranes of eukaryotes, including the endomembrane system (ES), define a space that contains the nucleus (N) and cytoplasm. Chloroplasts (C) and mitochondria (M), however, lie outside this space. Diagram also shows uniquely eukaryotic flagellum (F) anchored by a basal body (B). (Adapted from a figure by Max Taylor)
Merezhkovsky was right. Half a century later, so was Margulis. And the humble cyanobacteria take on a new importance as the source of photosynthesis in plants and algae. When you admire the greenery of a tropical forest, you are seeing blue-greens propelled to unprecedented ecological success by hitchhiking in a protozoan.
How can one cell become an integral part of another? The first requirement is straightforward: the host must not digest its guest. A cyanobacterial symbiont must have generated some product that inhibited release of its host’s digestive enzymes. The substance was sugar, leaked from the endosymbiont and absorbed by the cell that surrounded it. Successful host cells facilitated photosynthetic sugar production by ensuring a steady supply of carbon dioxide and nutrients to the symbiont. Through this metabolic exchange, a partnership emerged.
Alliances of this type are actually common in nature. For example, as Merezhkovsky recognized a century ago, reef corals harbor unicellular algae within their tissues, exchanging food for nutrients. The algae make it possible for corals to grow rapidly, but when the coral can’t hold up its end of the bargain, the algae depart, leaving their animal host pale—and doomed. In the present-day Caribbean Sea, coral “bleaching” linked to rising temperatures poses a serious threat to reef ecosystems.
Chloroplasts are manifestly
not
free to forsake their hosts. They have become anchored in place by a second type of exchange—one that is genetic rather than metabolic. Chloroplasts contain less than 10 percent of the DNA found in free-living cyanobacteria—in the transition from cell to organelle, the endosymbionts lost most of their genes.
How do chloroplasts function in the face of such genetic impoverishment? The answer is that proteins encoded by nuclear genes and synthesized in the cytoplasm are imported into the chloroplast. This requires proteins called chaperones that ferry molecules across the chloroplast
membranes. Chaperone proteins are ancient components of the cell’s machinery, originally formed to help new proteins fold properly and later co-opted as a transport service. Given this molecular support system, some cyanobacterial genes became redundant and were lost. And, in a process not well understood, some chloroplast genes actually migrated into the nucleus. In consequence, the photosynthetic factory came under nuclear control. From two distinct genealogical lineages, a new type of organism emerged.
Algae don’t all cluster together on the Tree of Life; instead, they scatter across several branches of the eukaryotic limb (
figure 8.2
). In principle, this spread can be explained in two different ways. Possibly, photosynthesis arose once, early in the evolutionary history of the Eucarya, and was later lost in some lineages, including our own. The alternative is that photosynthesis came to eukaryotes several times, by
repeated
symbiotic events. These two hypotheses make predictions that can be tested by molecular sequence comparisons. If all algae descended from a single symbiosis, then evolutionary trees based on comparisons of chloroplast genes should show the same genealogical relationships as trees based on nuclear genes. They don’t. Comparison of
figure 8.3
, which shows a molecular phylogeny of chloroplast genes, with the tree of eukaryotic organisms depicted in
figure 8.2
tells us that endosymbiosis must have brought photosynthesis to eukaryotes half a dozen times. What’s more, the story has a twist.
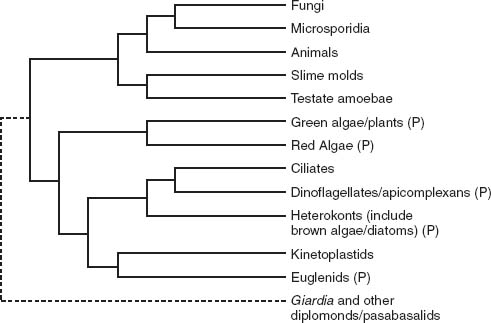
Figure 8.2.
A current hypothesis of genealogical relationships among eukaryotic organisms, based on molecular sequence comparisons of ten genes. Note the dotted line that connects diplomonads (which include
Giardia lamblia
) and parabasalids to the remainder of tree. This indicates the uncertainty surrounding the nature and composition of early branches on the tree. Groups with photosynthetic members marked by P. (Redrawn from a figure by Sandra Baldauf)