Life on a Young Planet (13 page)
Read Life on a Young Planet Online
Authors: Andrew H. Knoll

Faith shaken, but also, perhaps, faith restored. Elsewhere in southwestern Greenland, Minik Rosing, of the Geological Museum in Copenhagen, Denmark, has found a succession of metamorphosed shales some 160 feet thick. The rocks are more than 3.7 billion years old, are assuredly sedimentary, and contain abundant particles of graphite distributed much like the organic matter in younger shales. And once again, carbon isotopes suggest biological activity. In this case, however, Rosing argues convincingly that his graphites formed by the heating of
organic
matter, not the alteration of older minerals. Biology provides the simplest explanation for the chemistry in Rosing’s rocks—but a second line of evidence would be reassuring.
At present, our knowledge of Archean life and environments is both frustrating and exhilarating—frustrating because we are certain of so little, but exhilarating because we know anything at all. It is stimulating, as well, because the companion of ignorance is opportunity.
Some of the biggest questions focus on what came before Warrawoona, or Barberton, or even Akilia. If the oldest sedimentary rocks we can identify provide hints of complex microorganisms, what kinds of cells lived still earlier? Indeed, how did biology arise in the first place?
__________
1
Archean
(the time interval before the Proterozoic) and
Archaea
(a major branch on Tree of Life) both derive from the Greek
archaios
, meaning “ancient.” Beyond that, however, the terms have nothing to do with one another. The similar names do not imply that the Archean Eon was the Age of Archaea. It’s just that geologists and biologists don’t talk much to each other.
5 | The Emergence of Life |
Life was forged by the same physical and chemical processes that shaped our planet’s crust and oceans. Life is different, however, because it can undergo Darwinian evolution. Natural selection has played a key role in the evolution of plants and animals; early in our planet’s history, it also directed the chemical evolution that made life possible. In general terms, we understand how biological molecules might have evolved from simpler precursors present on the early Earth. But how proteins, nucleic acids, and membranes came to interact so intricately remains a mystery.
I
N A WELL-KNOWN
travelers’ tale, an explorer from the West treks up an Eastern mountain in search of a venerated sage. Finding the wise man in his aerie, and eager to show him a thing or two, the explorer asks, “What lies beneath the mountains we see around us?” “The mountains, the valleys, and everything else on the Earth rides on the back of a giant turtle,” replies the wise man. “What, then, lies beneath the turtle?” continues the traveler, sensing that his host has taken the bait. “Why, another turtle,” says the sage. “And beneath that?” “Another turtle.” “And then?” “Yet another turtle.” And so it goes, until the wise man, exasperated at last by his thickheaded guest, cries, “Don’t you see? It’s turtles all the way down!”
Biology has a “turtles all the way down” problem of its own. In
The Origin of Species
, Darwin hypothesized that new species arise by the modification of old—that the raw material of life is life. Louis Pasteur, Darwin’s great Parisian contemporary, went a step further. In his decisive refutation of spontaneous generation, the long-held view that life
can arise de novo from nonliving materials, Pasteur declared with Latin economy that “
omne vivum ex viva
.” Life springs
always
from life.
In science, answers provoke new questions, and so it isn’t surprising that in resolving two of biology’s greatest conundrums, Darwin and Pasteur laid bare its most profound mystery. Perhaps life has sprung only from life for the past four billion years, but sometime, somewhere, in the earliest days of our planet, our first ancestors had to arise from something else.
1
Rational thought about the origin of life actually predates both Darwin and Pasteur. For example, in 1804, before his famous grandson was even born, Erasmus Darwin captured the essence of biological history in verse:
Organic life beneath the shoreless waves
Was born and nurs’d in ocean’s pearly caves;
First forms minute, unseen by spheric glass,
Move on the mud, or pierce the watery mass;
These, as successive generations bloom,
New powers acquire and larger limbs assume;
Whence countless groups of vegetation spring,
And breathing realms of fin and feet and wing.
The younger Darwin knew these lines as he pondered natural selection, and he may have recalled them again in 1871 when he wrote to Benjamin Hooker about the ultimate origin of species:
It is often said that all the conditions for the first production of a living organism are now present, which could ever have been present. But if (and oh! What a big if!) we could conceive in some warm little pond, with all sorts of ammonia and phosphoric salts, light, heat, electricity, &c., present, that a proteine [
sic
] compound was chemically formed ready to undergo still more complex changes, at the present day such matter would be instantly devoured or absorbed, which would not have been the case before living creatures were formed.
In that letter, in a half dozen lines of conversational prose, Darwin outlined the central idea that has guided scientific thinking about the
origin of life ever since. The energy of nature drives simple molecules to combine and recombine, building chemical complexity until a system emerges that can replicate itself. The idea is powerful and intuitively appealing—life, seemingly so distinct from water and rock, arose from the same planetary processes that shaped Earth’s physical features. The question is how to test it.
The oldest sedimentary rocks we know of already contain at least a scrappy signature of biology, so we can’t recover a direct record of life’s origins from geology. The alternative is to devise laboratory experiments that allow us to evaluate the plausibility of hypothesized steps along the road to life. We can’t know with historical certainty whether any specific reaction played a role in the emergence of organisms, but we can seek to understand, in general terms, how chemical reactions on the early Earth could have made biology possible.
Sugars were synthesized from formaldehyde precursors as early as 1861, but only with an ingenious experiment conducted by Stanley Miller in 1953 did experimental research on the origin of life take flight. Working in the University of Chicago laboratory of Nobel laureate Harold Urey, Miller asked whether lightning could have synthesized the raw materials for life as it ripped through the primordial atmosphere. Others had thought about this question—Russian chemist Alexander Oparin and British biologist J.B.S. Haldane had both written penetrating essays on the origin of life in the 1920s—but Miller did more than cogitate. He filled a glass vessel with a mixture of methane, ammonia, hydrogen gas, and water vapor—judged by Urey to approximate Earth’s earliest atmosphere—and then repeatedly ran a spark though the vessel. Within a few days, the flask changed color, tinted reddish brown by a film on its inner surface. When Miller analyzed the fluid from which this gunk had precipitated, he found a variety of organic compounds, including amino acids, the building blocks of proteins.
In one remarkable experiment, Miller jump-started research on life’s origins. Powered by the energy of nature, simple gas mixtures could give rise to molecules of biological relevance and complexity. Amino acids and other biologically interesting compounds occur in carbonaceous meteorites and do so in proportions strikingly similar to those generated by Miller. Thus, what happened in Miller’s flask was not
some esoteric reaction likely to occur only in the laboratory, but rather a chemistry found widely in our solar system and beyond.
But, as ever, the answers provided by Miller’s simulation prompted new questions. Will any combination of primordial reactants do, or do biologically interesting molecules form only when you get the recipe right? Miller himself answered this one; the recipe matters a lot. Miller-Urey synthesis yields diverse and abundant organic molecules only when the ratio of hydrogen to carbon atoms in the gas mixture is at least four to one. That means that the chemistry in Miller’s beaker could have been important on the early Earth only if the primordial atmosphere was strongly reducing—devoid of oxygen and rich in hydrogen, methane, and/or ammonia. As discussed in
chapter 4
, most people agree that oxygen was scarce when Earth was young, but beginning with UCLA geochemist William Rubey in the 1950s, many workers have also come to believe that our planet’s earliest air was only weakly reducing, a mixture dominated by CO
2
and nitrogen gas rather than methane and ammonia. If the early atmosphere was indeed so weakly reducing, we need to look elsewhere for the kilns where biology’s bricks were made. Where might we look? More fundamentally, what should we be looking for?
Modern cells share a number of key features (
figure 5.1
). All have membranes that line the cell’s exterior and regulate molecular traffic into and out of the cytoplasm. Cells also synthesize proteins that catalyze chemical reactions or provide structural support.
2
And cells maintain a chemical library of information in the form of DNA. Critically important, membranes, proteins, and DNA continually interact within the cell. An impressive arsenal of proteins enables cells to consult the DNA library, replicate it in toto, or transcribe portions of it into RNA messages that provide blueprints for the formation of more proteins. The translation of RNA messages takes place in ribosomes, chemical factories made of protein and RNA woven together. Organisms also grow and reproduce, requiring that they take up materials and energy from their surroundings—metabolism employs still more proteins, some of them embedded in the cell’s membranes.
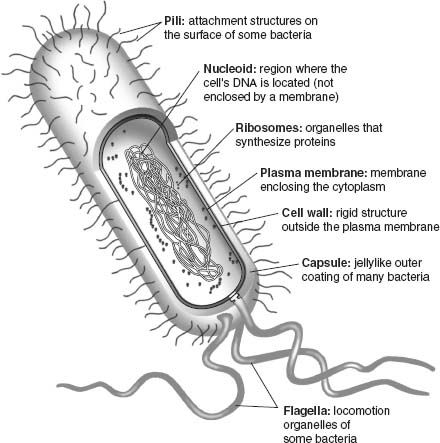
Figure 5.1.
The structure and function of a bacterial cell. RNA messages are transcribed from DNA within the nucleoid; these RNA messages are subsequently translated into proteins in chemical factories called ribosomes; cellular metabolism is carried out by pigments and proteins embedded in the cell’s membranes. (Reproduced with permission from N. A. Campbell and J. B. Reece,
Biology
, Sixth Edition. Copyright © 2002 by Pearson Education Inc.)
Even the simplest living organisms, then, are tremendously sophisticated molecular machines. The earliest life-forms had to be much, much simpler. We need to think about a family of molecules simple enough to form by physical processes, yet sufficiently complicated to lay the evolutionary groundwork for living cells. Such molecules would have contained information and structure sufficient to replicate themselves and, eventually, to direct the synthesis of other compounds that could catalyze
replication with increasing efficiency. And molecules able to initiate an evolutionary trajectory through which life could wean itself from the physical processes that gave it birth, synthesizing molecules needed for growth rather than incorporating them from the ambient environment, and tapping chemical or solar energy to fuel the workings of the cell.
RNA holds a special place in this line of thinking (
figure 5.2
). Long known for its functions in the translation of DNA into protein, RNA once seemed to be biology’s handmaiden, a mere go-between in a molecular drama dominated by DNA and proteins. As early as 1968, however, Nobel laureate Francis Crick envisioned a much grander role for RNA in life’s earliest history. “Possibly,” he mused, “the first ‘enzyme’ was an RNA molecule with replicase properties.” At the time it was written, Crick’s speculation probably struck many as daft. By the mid-1980s, however, it proved prophetic.
Crick’s prophecy was fulfilled in Thomas Cech’s lab at the University of Colorado. In studies of the ciliate protozoan
Tetrahymena thermophila
, Cech and his students had found that RNA destined for use in ribosomes was modified between the time of its transcription and the moment it glommed onto ribosomal proteins. Somehow, molecular surgeons snipped away an unwanted segment of the RNA molecule and then neatly spliced the remaining pieces back together. What enzymes, Cech asked, catalyzed this reaction?
Cech’s team began by purifying the unedited RNA sequence. Then they added it to a solution of proteins extracted from the ciliate’s nucleus. Not surprisingly, cutting and pasting of the RNA proceeded just as it does in the cell—the molecular catalyst was somewhere in the beaker. All good experiments, however, include controls designed to make sure that observed results aren’t produced by circumstances other than those being tested, so the team prepared additional test tubes with RNA but no proteins. And that’s where the surprise came. When Cech inspected the controls, he found that the RNA editing took place
even when proteins were absent
. The control experiment, usually the most mundane part of laboratory research, led Cech’s team to an electrifying conclusion: the RNA had excised the snippet by itself and spliced itself back together again. RNA could store information like DNA
and
catalyze reactions like proteins.