Life on a Young Planet (14 page)
Read Life on a Young Planet Online
Authors: Andrew H. Knoll

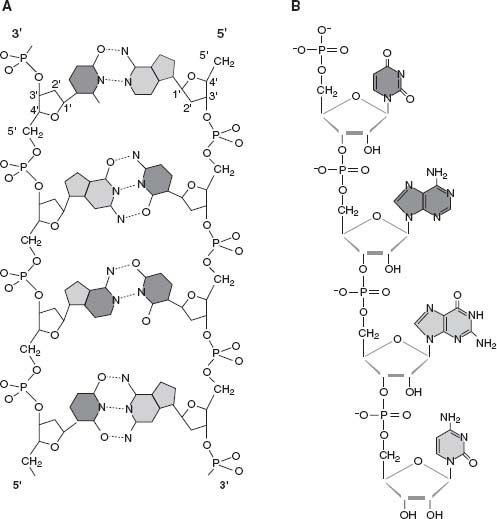
Figure 5.2.
The molecular structures of DNA and RNA. (a) DNA, showing how a chemical backbone of phosphate and deoxyribose sugar combines with four bases that provide both molecular information and the bonds that link two strands into a double helix. (b) RNA, built from ribose sugar, phosphate, and four bases (one of which differs from its DNA counterpart). (a) Adapted from illustration by Irving Geis from R. E. Dickerson (1983). The DNA helix and how it is read,
Scientific American
249: 97–112; rights owned by the Howard Hughes Medical Institute. Not to be reproduced without permission; (b) reproduced with permission from S. Freeman, 2002,
Biological Science
, Prentice Hall)
The discovery of RNA enzymes, or
ribozymes
, made independently at about the same time by Yale biochemist Sidney Altman, had—dare I say it—a catalytic effect on thinking about life’s origins. As philosopher of biology Iris Fry put it, this remarkable molecule emerged as “both chicken and egg” in the riddle of life’s origins. In 1986, Walter Gilbert, a Harvard
colleague, crafted a short but stimulating essay whose title, “The RNA World,” came to symbolize a conceptual way station in the evolution of biochemical complexity. As Gilbert saw it, RNA was the information-rich molecule that could form by self-organization and catalyze its own replication. Later, as life matured, evolution introduced a division of labor, with double helices of DNA providing a more stable library, while intricately folded proteins took over most catalytic functions.
This road to biology is tremendously appealing, but it contains a number of speed bumps. First and most important is the difficulty of making RNA under plausible prebiotic conditions. RNA molecules contain a backbone of the five-carbon sugar ribose and phosphate (PO
4
3−
) joined together in a chain (
figure 5.2
). Four bases, chemical compounds built from rings of carbon and nitrogen, attach to the sugars, imparting molecular information. The bases can be synthesized easily enough—in 1961 Spanish biochemist Juan Oró showed that one of them, adenine, could form directly by combining five molecules of hydrogen cyanide (the modus operandi in many a detective story and likely present on the young Earth). Ribose, on the other hand, isn’t so easy to explain. As noted earlier, sugars can be synthesized from solutions containing formaldehyde (probably also present in our planet’s infancy), but ribose is only one of many products, and a minor one at that. The processes by which this sugar might have been thrust onto prebiotic center stage are not obvious. Worst of all, even if we could produce the right components, combining them to form nucleotides, the building blocks of nucleic acids, is daunting. To date, no one has figured out how to do it.
There is still another difficulty. Nucleotides are chiral molecules, which is to say that they come in two forms that are mirror images of each other—like your hands. RNA can be built from right-handed or left-handed nucleotides, but mixed chains won’t grow. How, then, could RNA—which in cells consists exclusively of right-handed nucleotides—have emerged from a fifty-fifty mixture of left- and right-handed building blocks? Again, no one knows.
The problems are so difficult that many researchers have given up on the idea that RNA was the primordial molecule of life. They suggest instead that prebiotic evolution began with molecules without “handedness” that are easier to synthesize and polymerize. Nonchiral molecules that form double helices like those of nucleic acids can indeed be generated with relative ease in the laboratory. Moreover, at least one of them, called peptide nucleic acid (
figure 5.3
), can direct the formation of its RNA complement, supporting the hypothesis that RNA could have replaced its primordial precursors later, in the course of evolution.
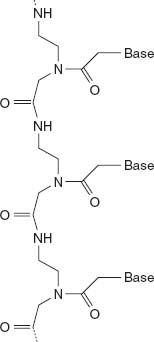
Figure 5.3.
The molecular structure of peptide nucleic acid, a nonchiral molecule, illustrating one possible route in the evolution of nucleic acids.
We have a better sense of how the RNA world might have operated once it burst onto the scene. Pioneering experiments in the laboratories of Jack Szostak and Jerry Joyce, at Harvard Medical School and the Scripps Research Institute, respectively, show how natural selection could have honed the function of RNA molecules. The experiments typically begin with millions of RNA strands generated randomly in the laboratory. Those showing weak ability to catalyze a specific reaction are selected and replicated repeatedly under conditions that introduce mutations into the replicates. A second round of selection ensues, followed by more replication. Repeated replication and selection yield functionally efficient ribozymes.
These experiments show that many types of RNA catalysis are possible; RNA provides just the sort of jack-of-all-trades molecule needed to get biology going. They also suggest that natural selection can generate
molecular order from disorder, and amplify weak biochemical function. If Szostak, Joyce, and their colleagues are on the right track, evolution is not only the hallmark of biology, but a prerequisite for life.
This, in turn, highlights a cardinal feature of nucleic acid replication at the dawn of life. To quote Ronald Reagan, “mistakes were made.” As early RNA molecules copied themselves, errors crept in so that molecular daughters included sequence variations not found in their parents. This variation supplied the raw material for chemical evolution on the early Earth, and it has fueled biological evolution ever since that time.
As in many other spheres of life, the Goldilocks rule applies. If the error rate of RNA (and later, DNA) replication is too high, successful variants can’t be perpetuated in succeeding generations. If, on the other hand, it is too low, evolution cannot continue. That actual error rates are “just right” may seem a remarkable coincidence, but it isn’t—it results from natural selection at the molecular level. Limited sloppiness is an evolutionary virtue.
We can, thus, envision a central role for RNA in nascent life. Experiments show that in the presence of mineral catalysts, nucleotides can join together to form RNA (although nucleotides, themselves, have not yet been built from scratch), and relatively short RNA molecules can direct their own replication. From the pool of sequence variation created by copying errors, natural selection can amplify those sequences that function best, those that replicate themselves a bit faster or with fewer errors than their molecular neighbors.
It turns out that much the same can be said for proteins. As Stanley Miller demonstrated, amino acids form readily under at least some prebiotic conditions, and, like nucleic acids, they can join together to form peptides, the amino acid chains that fold to form functioning proteins. Reza Gadhiri and his colleagues at the Scripps Research Institute have even generated peptides that catalyze their own replication. Thus, depending on environmental conditions, nucleic acid
and
protein precursors could have evolved in primeval oceans.
Regardless of which (if either) set of molecules came first, however, the most formidable problem in primordial evolution must be the emergence of systems in which proteins and nucleic acids
interact
, each ensuring the survival of the other. Freeman Dyson, a renowned physicist who has thought deeply about life’s origins, posited that life actually
began twice, once via the RNA route and again by way of proteins. Cells with interacting proteins and nucleic acids subsequently arose by protobiological merger. The idea isn’t crazy. As will be clear by the end of this book, innovation by alliance is a major theme in evolution.
Viewed as a chicken-and-egg problem, Dyson’s proposal has obvious attractions. But the issue is more complicated than that, because it must also be approached as a question of locks and keys. Molecular cross talk between nucleic acids and proteins is mediated by the genetic code, a set of chemical correspondences that allows the molecular language of nucleotides to be translated into the amino acid chains of proteins. Was the code a “frozen accident,” as suggested by Francis Crick, and if so what was the nature of the accident? Alternatively, do chemical rules underlie the molecular correspondences, and if so, what are they? The origin of the genetic code, and with it the emergence of biochemically complex life, remains biology’s mystery of mysteries.
One further riddle remains to be explored. Metabolism weaned life from the physical processes that gave it birth, and the metabolic pathways introduced in
chapter 2
have perpetuated biology for some 4 billion years. How does the evolution of metabolism fit into the scenario outlined in previous paragraphs?
Membranes made of phospholipids (molecules with a “head” of phosphate and organic carbon and two long “tails” of fatty acids) serve both to separate cells from their physical surroundings and to direct the metabolic traffic of ions, molecules, and energy. Contemporary phospholipids may, like DNA, have arisen during the course of early biological evolution; however, simpler molecules able to assemble spontaneously into membranous vesicles may well have existed in the primeval ocean; spherical membranes made from lipidlike compounds in meteorites look more than a little like cells.
Possibly, the linkage of metabolism and replication began with the packaging of RNA (or protein and RNA) molecules inside primitive membranes. A simple experiment conducted by University of California biochemist David Deamer illustrates how this might have occurred. Deamer took a mixture of DNA and lipid vesicles and repeatedly wet and dried it. When the mixture dried, the molecules formed a layered “sandwich” on the bottom of the reaction flask. During subsequent
wetting, the lipids reconstituted their spherical vesicles, but now, some of the DNA strands were
inside
the vesicles. This suggests that the structural association of lipids, proteins, and nucleic acids could have arisen spontaneously on the early Earth. Moreover, Deamer and his colleagues were able to synthesize RNA within vesicles, using nucleotides imported across the bounding membrane. This provides a faint glimmer of how metabolism and replication could have become linked. And once again, the Goldilocks rule is in order.
Membrane function depends strongly on the length of the fatty acid “tails” in constituent phospholipids. If they are too short, the membrane will be so leaky that it won’t work. If they are too long, nothing can pass through the membrane, an equally fatal circumstance. Thus, the first membranes, at least the first ones that worked, must have formed spontaneously with lipid “tails” long enough to keep large polymers on the inside, but short enough to allow smaller molecules to pass in and out of the vesicle.
The key to metabolic integration must, of course, have been the evolution of nucleic acid sequences that coded for proteins able to direct membrane synthesis. Once the membrane came under cellular control, it, too, became subject to natural selection, leading (again) to molecular division of labor. The phospholipid “tails” grew longer, prohibiting passage of all but a few molecules, such as water and simple gases. At the same time, proteins became embedded in the phospholipid matrix, providing specialized gates and channels that admitted ions, molecules, and energy in a controlled fashion. Like nucleic acids, then, membranes appear to have evolved from simple, unspecialized structures formed by chemical processes to sophisticated, specialized systems constructed by the cell.
The scenario outlined in previous paragraphs begins with nucleic acids or their molecular forebears and expands to include proteins, membranes, and ultimately, metabolism. Some scientists, however, believe that things happened the other way around—that life
began
with metabolism and subsequently invented nucleic acids and proteins.
Gunther Wächtershäuser, a Munich chemist and patent lawyer, has argued the case for life’s metabolic origin with particular clarity and vigor. Conventional prebiotic syntheses, he notes, work only when
environmental conditions are just right. Considering that conditions may not have been right in the primordial oceans, Wächtershäuser concludes that life must have gotten started some other way in some other place. The place he advocates is a hydrothermal spring, like those found in the Warrawoona seaway or along present-day midocean ridges. In these settings, hydrogen sulfide emitted from vents can react with iron monosulfide to form pyrite—great chimneys of pyrite still form at the mouths of deep-sea hydrothermal vents. The reaction yields both energy and chemical reducing power (in the form of hydrogen), powering, in Wächtershäuser’s scenario, the fixation of carbon dioxide (or carbon monoxide) to form organic compounds on the surfaces of growing pyrite crystals.