From Eternity to Here (6 page)

THE ARROW OF TIME
There is a good reason why reversing the relative direction of time is an effective tool of the imagination: In the actual, non-imaginary world, it never happens. Time has a direction, and it has the
same
direction for everybody. None of us has met a character like the White Queen, who remembers what we think of as “the future” rather than (or in addition to) “the past.”
What does it mean to say that time has a direction, an arrow pointing from the past to the future? Think about watching a movie played in reverse. Generally, it’s pretty clear if we are seeing something running the “wrong way” in time. A classic example is a diver and a pool. If the diver dives, and then there is a big splash, followed by waves bouncing around in the water, all is normal. But if we see a pool that starts with waves, which collect into a big splash, in the process lifting a diver up onto the board and becoming perfectly calm, we know something is up: The movie is being played backward.
Certain events in the real world always happen in the same order. It’s dive, splash, waves; never waves, splash, spit out a diver. Take milk and mix it into a cup of black coffee; never take coffee with milk and separate the two liquids. Sequences of this sort are called
irreversible processes
. We are free to imagine that kind of sequence playing out in reverse, but if we actually see it happen, we suspect cine matic trickery rather than a faithful reproduction of reality.
Irreversible processes are at the heart of the arrow of time. Events happen in some sequences, and not in others. Furthermore, this ordering is perfectly consistent, as far as we know, throughout the observable universe. Someday we might find a planet in a distant solar system that contains intelligent life, but nobody suspects that we will find a planet on which the aliens regularly separate (the indigenous equivalents of) milk and coffee with a few casual swirls of a spoon. Why isn’t that surprising? It’s a big universe out there; things might very well happen in all sorts of sequences. But they don’t. For certain kinds of processes—roughly speaking, complicated actions with lots of individual moving parts—there seems to be an allowed order that is somehow built into the very fabric of the world.
Tom Stoppard’s play
Arcadia
uses the arrow of time as a central organizing metaphor. Here’s how Thomasina, a young prodigy who was well ahead of her time, explains the concept to her tutor:
THOMASINA: When you stir your rice pudding, Septimus, the spoonful of jam spreads itself round making red trails like the picture of a meteor in my astronomical atlas. But if you need stir backward, the jam will not come together again. Indeed, the pudding does not notice and continues to turn pink just as before. Do you think this odd?
SEPTIMUS: No.
THOMASINA: Well, I do. You cannot stir things apart.
SEPTIMUS: No more you can, time must needs run backward, and since it will not, we must stir our way onward mixing as we go, disorder out of disorder into disorder until pink is complete, unchanging and unchangeable, and we are done with it for ever. This is known as free will or self-determination.
23
The arrow of time, then, is a brute fact about our universe. Arguably
the
brute fact about our universe; the fact that things happen in one order and not in the reverse order is deeply ingrained in how we live in the world. Why is it like that? Why do we live in a universe where
X
is often followed by
Y
, but
Y
is never followed by
X
?
The answer lies in the concept of “entropy” that I mentioned above. Like energy or temperature, entropy tells us something about the particular state of a physical system; specifically, it measures how disorderly the system is. A collection of papers stacked neatly on top of one another has a low entropy; the same collection, scattered haphazardly on a desktop, has a high entropy. The entropy of a cup of coffee along with a separate teaspoon of milk is low, because there is a particular orderly segregation of the molecules into “milk” and “coffee,” while the entropy of the two mixed together is comparatively large. All of the irreversible processes that reflect time’s arrow—we can turn eggs into omelets but not omelets into eggs, perfume disperses through a room but never collects back into the bottle, ice cubes in water melt but glasses of warm water don’t spontaneously form ice cubes—share a common feature: Entropy
increases
throughout, as the system progresses from order to disorder. Whenever we disturb the universe, we tend to increase its entropy.
A big part of our task in this book will be to explain how the single idea of entropy ties together such a disparate set of phenomena, and then to dig more deeply into what exactly this stuff called “entropy” really is, and why it tends to increase. The final task—still a profound open question in contemporary physics—is to ask why the entropy was so low in the past, so that it could be increasing ever since.
FUTURE AND PAST VS. UP AND DOWN
But first, we need to contemplate a prior question: Should we really be surprised that certain things happen in one direction of time, but not in the other? Who ever said that everything should be reversible, anyway?
Think of time as a label on events as they happen. That’s one of the ways in which time is like space—they both help us locate things in the universe. But from that point of view, there is also a crucial difference between time and space—directions in space are created equal, while directions in time (namely, “the past” and “the future”) are very different. Here on Earth, directions in space are easily distinguished—a compass tells us whether we are moving north, south, east, or west, and nobody is in any danger of confusing up with down. But that’s not a reflection of deep underlying laws of nature—it’s just because we live on a giant planet, with respect to which we can define different directions. If you were floating in a space suit far away from any planets, all directions in space would truly be indistinguishable—there would be no preferred notion of “up” or “down.”
The technical way to say this is that there is a
symmetry
in the laws of nature—every direction in space is as good as every other. It’s easy enough to “reverse the direction of space”—take a photograph and print it backward, or for that matter just look in a mirror. For the most part, the view in a mirror appears pretty unremarkable. The obvious counterexample is writing, for which it’s easy to tell that we are looking at a reversed image; that’s because writing, like the Earth, does pick out a preferred direction (you’re reading this book from left to right). But the images of most scenes not full of human creations look equally “natural” to us whether we see them directly or we see them through a mirror.
Contrast that with time. The equivalent of “looking at an image through a mirror” (reversing the direction of space) is simply “playing a movie backward” (reversing the direction of time). And in that case, it’s easy to tell when time has been inverted—the irreversible processes that define the arrow of time are suddenly occurring in the wrong order. What is the origin of this profound difference between space and time?
While it’s true that the presence of the Earth beneath our feet picks out an “arrow of space” by distinguishing up from down, it’s pretty clear that this is a local, parochial phenomenon, rather than a reflection of the underlying laws of nature. We can easily imagine ourselves out in space where there is no preferred direction. But the underlying laws of nature do not pick out a preferred direction of time, any more than they pick out a preferred direction in space. If we confine our attention to very simple systems with just a few moving parts, whose motion reflects the basic laws of physics rather than our messy local conditions, there is no arrow of time—we
can’t
tell when a movie is being run backward. Think about Galileo’s chandelier, rocking peacefully back and forth. If someone showed you a movie of the chandelier, you wouldn’t be able to tell whether it was being shown forward or backward—its motion is sufficiently simple that it works equally well in either direction of time.
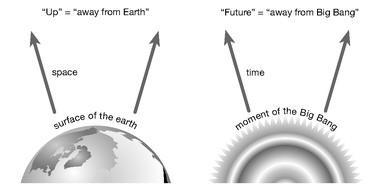
Figure 5:
The Earth defines a preferred direction in space, while the Big Bang defines a preferred direction in time.
The arrow of time, therefore, is not a feature of the underlying laws of physics, at least as far as we know. Rather, like the up/down orientation space picked out by the Earth, the preferred direction of time is also a consequence of features of our environment. In the case of time, it’s not that we live in the spatial vicinity of an influential object; it’s that we live in the temporal vicinity of an influential event: the birth of the universe. The beginning of our observable universe, the hot dense state known as the Big Bang, had a very low entropy. The influence of that event orients us in time, just as the presence of the Earth orients us in space.
NATURE’S MOST RELIABLE LAW
The principle underlying irreversible processes is summed up in the Second Law of Thermodynamics:
The entropy of an isolated system either remains constant or increases with time.
(The First Law states that energy is conserved.
24
) The Second Law is arguably the most dependable law in all of physics. If you were asked to predict what currently accepted principles of physics would still be considered inviolate a thousand years from now, the Second Law would be a good bet. Sir Arthur Eddington, a leading astrophysicist of the early twentieth century, put it emphatically:
If someone points out to you that your pet theory of the universe is in disagreement with Maxwell’s equations [the laws of electricity and magnetism]—then so much the worse for Maxwell’s equations. If it is found to be contradicted by observation—well, these experimentalists do bungle things sometimes. But if your theory is found to be against the Second Law of Thermodynamics I can give you no hope; there is nothing for it but to collapse in deepest humiliation.
25
C. P. Snow—British intellectual, physicist, and novelist—is perhaps best known for his insistence that the “Two Cultures” of the sciences and the humanities had grown apart and should both be a part of our common civilization. When he came to suggest the most basic item of scientific knowledge that every educated person should understand, he chose the Second Law:
A good many times I have been present at gatherings of people who, by the standards of the traditional culture, are thought highly educated and who have with considerable gusto been expressing their incredulity at the illiteracy of scientists. Once or twice I have been provoked and have asked the company how many of them could describe the Second Law of Thermodynamics, the law of entropy. The response was cold: it was also negative. Yet I was asking something which is about the scientific equivalent of: “Have you read a work of Shakespeare’s?”
26
I’m sure Baron Snow was quite the hit at Cambridge cocktail parties. (To be fair, he did later admit that even physicists didn’t really understand the Second Law.)
Our modern definition of entropy was proposed by Austrian physicist Ludwig Boltzmann in 1877. But the concept of entropy, and its use in the Second Law of Thermodynamics, dates back to German physicist Rudolf Clausius in 1865. And the Second Law itself goes back even earlier—to French military engineer Nicolas Léonard Sadi Carnot in 1824. How in the world did Clausius use entropy in the Second Law without knowing its definition, and how did Carnot manage to formulate the Second Law without even using the concept of entropy at all?
The nineteenth century was the heroic age of thermodynamics—the study of heat and its properties. The pioneers of thermodynamics studied the interplay between temperature, pressure, volume, and energy. Their interest was by no means abstract—this was the dawn of the industrial age, and much of their work was motivated by the desire to build better steam engines.
Today physicists understand that heat is a form of energy and that the temperature of an object is simply a measure of the average kinetic energy (energy of motion) of the atoms in the object. But in 1800, scientists didn’t believe in atoms, and they didn’t understand energy very well. Carnot, whose pride was wounded by the fact that the English were ahead of the French in steam engine technology, set himself the task of understanding how efficient such an engine could possibly be—how much useful work could you do by burning a certain amount of fuel? He showed that there is a fundamental limit to such extraction. By taking an intellectual leap from real machines to idealized “heat engines,” Carnot demonstrated there was a best possible engine, which got the most work out of a given amount of fuel operating at a given temperature. The trick, unsurprisingly, was to minimize the production of waste heat. We might think of heat as useful in warming our houses during the winter, but it doesn’t help in doing what physicists think of as “work”—getting something like a piston or a flywheel to move from place to place. What Carnot realized was that even the most efficient engine possible is not perfect; some energy is lost along the way. In other words, the operation of a steam engine is an irreversible process.