Everything Kids' Magical Science Experiments Book
Read Everything Kids' Magical Science Experiments Book Online
Authors: Tim Robinson
Tags: #epub, ebook


THE
EVERYTHING
®
KIDS'
MAGICAL
SCIENCE EXPERIMENTS
BOOK
Dazzle your friends
and family with dozens
of science tricks!
Tom Robinson
Puzzles by Beth L. Blair

Copyright ©2007, F+W Publications, Inc. All rights reserved. This book, or parts thereof,
may not be reproduced in any form without permission from the publisher; exceptions are made
for brief excerpts used in published reviews and photocopies made for classroom use.
An Everything® Series Book.
Everything® and
everything.com
® are registered trademarks of F+W Publications, Inc.
Published by Adams Media, an F+W Publications Company
57 Littlefield Street, Avon, MA 02322. U.S.A.
www.adamsmedia.com
ISBN 10: 1-59869-426-X
ISBN 13: 978-1-59869-426-0 (paperback)
ISBN 13: 978-1-60550-244-1 (EPUB)
Printed in the United States of America.
J I H G F E D C B A
This publication is designed to provide accurate and authoritative information with regard to the subject matter covered. It is sold with the understanding that the publisher is not engaged in rendering legal, accounting, or other professional advice. If legal advice or other expert assistance is required, the services of a competent professional person should be sought.
â From a
Declaration of Principles
jointly adopted by a Committee of the American Bar Association and a Committee of Publishers and Associations
Many of the designations used by manufacturers and sellers to distinguish their products are claimed as trademarks. When those designations appear in this book and Adams Media was aware of a trademark claim, the designations have been printed with initial capital letters.
Note:
All activities in this book should be performed with adult supervision. Likewise common sense and care are essential to the conduct of any and all activities described in this book. Parents or guardians should supervise children. Neither the author nor the publisher assumes any responsibility for any injuries or damages arising from any activities or outings.
Cover illustrations by Dana Regan. Interior illustrations by Kurt Dolber. Puzzles by Beth L. Blair.
This book is available at quantity discounts for bulk purchases. For information, please call 1-800-289-0963.
See the entire Everything® series at
www.everything.com
.
| EDITORIAL | PRODUCTION |
|---|---|
| Innovation Director: Paula Munier | Director of Manufacturing: Susan Beale |
| Editorial Director: Laura M. Daly | Production Project Manager: Michelle Roy Kelly |
| Associate Copy Chief: Sheila Zwiebel | Prepress: Erick DaCosta, Matt LeBlanc |
| Acquisitions Editor: Kerry Smith | Interior Layout: Heather Barrett, Brewster Brownville, Colleen Cunningham, Jennifer Oliveira |
| Development Editor: Brett Palana-Shanahan | Â |
| Production Editor: Casey Ebert | Â |
For Matt and Megan

Chapter 1: Try It, You'll Like It!
Chapter 2: Running Hot and Cold
Chapter 3: Life in a Fun House
Chapter 4: Water, Water Everywhere
Chapter 5: The Incredible Machine
W
ould you pay a lot of money to see a scientist do experiments? Probably not. Now if it were a magician, that might be a different story. Magicians awe people. They do things that seem impossible. Their tricks defy any explanation and as hard as the audience tries to figure out how they work, they often cannot. For many, science works in the same way. They see the strange and the unexpected, and when they can't explain it, they assume that science is magic. But there is one big difference between science and magic. That difference is what makes science something everyone can do, while magic is usually left to the professionals.
What is this one difference between the two disciplines? A scientist wants to know why things work the way they do. And so does a magician. The difference is that a scientist wants others to know, also. As a result, when a scientist learns an answer to a question, he often writes a report, explaining what his original question was, what he did to test the question, and what the answer was.
Magicians never reveal their secrets. That is, magicians want you to believe that what they are doing is magic, that it cannot be explained in simple terms. Scientists, on the other hand, believe that everything has an explanation, and are happy to share that explanation with anyone who will listen.
In this book, you will be exploring a number of “magical” experiments. Many produce results that are unexpected, surprising, and initially unexplainable. But this is a science book, not a magic book. You will be shown the science behind the apparent magic, so that you can pass it along to others. You will also be challenged to take your newfound knowledge to a deeper level, by asking questions that will let you explore the concepts you are learning. At the end of each chapter, you will find an idea for a science-fair project, which is really just a more complete science experiment that you could perform over the course of a few days, weeks, or months.
As you begin, think about science as the search for explanations of common and not-so-common experiences. By the time you finish this book, you should clearly see the difference between the magic of a trained professional and the science you can do in your own home.
The experiments are organized into common themes. Each experiment is preceded by a question, which then leads to a process known as the Scientific Method. This process allows questions to lead to investigations, which lead to results, conclusions, and possibly, new questions.
The Scientific Method includes five important parts:
- Ask a question about something that happens in the world around you.
- Make up a possible explanation for this event. This is called a
hypothesis. - Design an experiment to test your hypothesis.
- Complete the experiment and record your results.
- Analyze your results and use them to come to a conclusion about your hypothesis.
Scientists have been using this method for centuries to explore the magical and mysterious things they see around them. Now it's your turnâlet the adventure begin!
Demo version limitation
Running Hot and Cold

W
hat is summer like where you live? Is it hot and humid? Do you wish you had a personal air conditioner you could carry with you wherever you go? What about winter? Are the streets continually covered with snow and ice, and do the cloudy, cold days seem to last forever?
One of the most remarkable changes that takes place over the course of a year, no matter where you live, is the change in seasons. From the hot, summer days, to the cold, wintry nights, the changes in hot and cold weather always make us take note of the changing seasons.
The act of heating something up or cooling it down often produces surprising results.

“We live in a society dependent on science and technology, in which hardly anyone knows anything about science and technology.”
â
Carl Sagan
In this chapter, you will explore the effects of heating and cooling different materials and the magic that happens when you do. In this chapter, you'll explore the magic of exploding ice, floating water, sharp string, and rising dough.
One of the well-known “facts” about heating and cooling materials is that when things heat up, they expand, and when they cool down, they shrink. As an example, if you were to try to open a jar of spaghetti sauce whose lid was tightly sealed, you might run the lid under hot water. The heat of the water causes the lid to expand just enough to make it easier to open the lid.
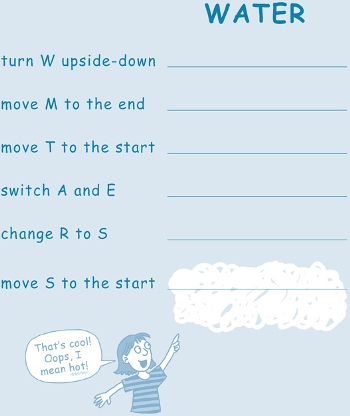
Add enough heat to cold water and it turns into something completely different â it seems like magic! Follow the directions below to see what you end up with. On the lines provided, write the letters you get after completing each step.
Similarly, you might notice little cracks in the sidewalks near your house. These are put there on purpose, and they are called expansion joints. In the summer, the concrete usually expands and the cracks are thin. But in the winter, the cracks tend to open wider as the concrete shrinks. But does cooling things always make them shrink?
Question: Can you “explode” a bottle of water by freezing it?
- A 500-mL bottle of drinking water
- Freezer
- Marking pen
- Stand the bottle on a counter and mark the height of the water. There should be a small amount of air between the surface of the water and the cap.
- Place the bottle upright in the freezer and let it sit for at least three hours.
- Take the bottle out of the freezer and notice how the water height has changed.
This seems to be a magical result, but it's not. Water is an unusual substance in that as it freezes, instead of shrinking, as you might expect it to do, it actually expands. This is because of the air that is stored in the water as a liquid. That air expands as the water freezes into ice, and that makes the ice take up more room than the water did. Your bottle may have been distorted slightly, as the ice caused the bottle to expand.

EXPANSION JOINT:
A crack or gap intentionally placed in objects like sidewalks and roads that allow the material to expand and shrink in different temperatures without causing the road or sidewalk to buckle.
One consequence of this process is that the same amount of water (H2O) takes up more space. The term that describes this relationship between amount of material and amount of space it takes up is
density
. The more material there is in the same space, the more dense the material is. On the other hand, if you have the same amount of something, but it takes up more space, it is less dense. When the water freezes and expands to form ice, the same amount of material takes up more space and therefore, it is less dense.
This change explains how ice cubes can float in a glass of water. Since ice is less dense than water, it floats. This is similar to how icebergs, which are really just huge, floating chunks of ice, can float in the ocean.
What do you think would happen if you heated up that bottle of water? You have to be careful, because if you heat it too much, the plastic bottle might melt. But here is another place you can see this result. If you have a thermometer at home, the kind you take your temperature with when you are not feeling well, you may be able to explore this. It needs to be the kind that is made of glass, with a thin line of red liquid showing the temperature. When you stick the thermometer in your mouth, the red liquid in the base of the thermometer expands and climbs up the tube until it stops at the temperature of your mouth.
Can you think of any other examples of liquids expanding or shrinking when you heat or cool them? What about objects that are solid? See if you can find five examples of objects that shrink or expand when heated or cooled.
This site explores the world of icebergs, and takes a look at the collision that caused the
Titanic
to sink in 1912:
http://oceanworld.tamu.edu/students/iceberg.

Salt is often used on roads and sidewalks as a de-icer. What it does is lower the freezing temperature of the ice. This means that ice made from pure water that has frozen at 32° Fahrenheit may turn to water at that same temperature when mixed with salt.


