Everything Kids' Magical Science Experiments Book (2 page)
Read Everything Kids' Magical Science Experiments Book Online
Authors: Tim Robinson
Tags: #epub, ebook


Ice can be slippery. In the winter, when it snows, the roads often turn icy and people have a hard time driving on it. If you have ever tried to ice skate, chances are, the first time you tried it you found out just how slick ice can be. Even picking up ice cubes can be difficult, as they tend to slip right out of your hands. So perhaps there is a better wayâdon't use your hands!
Question: Can you lift an ice cube without touching it?
- Several large ice cubes, taken directly from the freezer
- Salt
- String that can be cut into pieces
- Scissors
- Paper towel
- Place one ice cube on the paper towel.
- Cut a piece of string at least 12 inches long.
- Without touching the ice cube with your hands, try to lift the ice cube using only the string.
- Lay the string across the top of the ice cube, leaving several inches free on either side.
- Pour salt on the ice cube, covering the top of the ice and the string.
- Wait 3â5 minutes.
- Lift the string from both ends and watch the magic happen.

The freezing temperature of salt water is approximately 28°F. This is part of the reason why lakes and rivers, made from fresh water, tend to freeze more than bodies of salt water. Can you think of another possible reason?

MELTING POINT:
The temperature at which a solid melts to form a liquid. This is also commonly called the freezing point, as it is also the temperature of the reverse change at which a liquid freezes to form a solid.
By itself, the string cannot lift the ice cube. However, salt has a special relationship with ice. When you pour salt on the ice, the ice melts slightly, and then refreezes at a slightly lower temperature. Salt water has a lower melting point than pure water. When the ice refreezes, it “captures” the string under the surface of the ice. When you lift up on the string, the ice comes with it.
How much salt does it take to produce this effect? See if you can devise an experiment that will test to see how little salt you can use to still lift the ice cube. Alternately, you may want to try this experiment with ice at different temperatures. Try using ice taken straight from the freezer and compare the results to ice that has been sitting out on the counter for 5 minutes, 10 minutes, or more.
Also, if it snows where you live, the next time it happens, try pouring some salt on a small area of snow or ice and see whether or not the salt helps melt the ice.
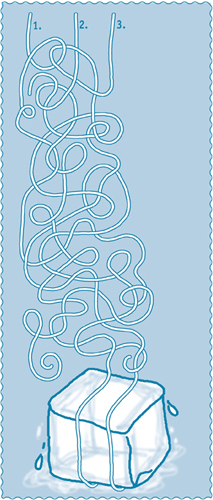
Follow the strings over and under. Which string will be able to lift the ice cube?
Question: Can you make water float on oil?
In this experiment, you will first demonstrate how it is possible to make water “float” on oil by cooling it to the point that it freezes. You see, when water freezes, it expands and becomes less dense. This change is usually enough to make the density of ice less than that of the cooking oil. You will pour cooking oil and colored water into a jar and allow them to settle until one floats on top of the other. Then you will place the jar in the freezer for several hours. What you see when you remove the jar from the freezer may surprise you.
Your next task will be to test other liquid combinations to see if freezing them changes their density enough to make “heavy” liquids float.
KIDS' LAB LESSONS
The factor that determines whether or not something will float is its density. For example, a piece of wood floats in a lake because the wood's density is less than that of water. However, if you were to drop a large boulder into the lake, it sinks quickly to the bottom. The boulder's density is greater than that of water.
If you were to pour some cooking oil into a jar containing water, the oil wouldn't mix with the water. It would appear to “float” or sit on top of the water. This is because the density of cooking oil is less than that of water. This is also why you tend to see rainbows of colors where oil or gasoline has spilled on wet pavement. The oil stays on top of the water because of its lower density.
When you freeze the water, however, its density decreases to the point that the ice will actually float on top of the oil, as if it were an ice cube in a very large drink.
- Large, clear, canning jar without a lid
- Measuring cups
- Food coloring
- 1 cup of tapwater
- 1 cup of cooking oil
- Other liquids for testing, such as:â Corn syrupâ Pancake syrupâ Sodaâ Molassesâ Milkâ Coffee
- Pour one cup of cooking oil into the canning jar.
- Mix a few drops of food coloring into one cup of water until the color is easy to observe.
- Slowly pour the colored water into the jar containing oil. Watch carefully to see how the water and oil interact. Do they mix, or do they stay separate?
- Take note of which liquid “floats” on top of the other.
- After clearing a spot in the freezer, place the jar in the freezer for several hours. Resist the temptation to sneak a peek while you wait.
- When sufficient time has passed, remove the jar from the freezer and observe any changes you see.
- Why don't you think the oil and water mixed when you placed them in the jar?

- Which liquid appeared to have the lowest density? How could you tell?

- What did you observe about your liquids when you removed the jar from the freezer? Did they look the same as when you placed them in the freezer?


- What do you predict will happen as the ice begins to melt? Let the jar sit in the room for a period of time and observe the changes in the two liquids as the ice melts. Describe what you observe.

