Your Inner Fish: A Journey Into the 3.5-Billion-Year History of the Human Body (12 page)
Read Your Inner Fish: A Journey Into the 3.5-Billion-Year History of the Human Body Online
Authors: Neil Shubin

Our particular brand of hardness, with teeth inside our mouths and bones inside our bodies, is an essential part of who we are. We can eat, move about, breathe, even metabolize certain minerals because of our hydroxyapatite-containing tissues. For these capabilities, we can thank the common ancestor we share with all fish. Every fish, amphibian, reptile, bird, and mammal on the planet is like us. All of them have hydroxyapatite-containing structures. But where did this all come from?
There is an important intellectual issue at stake here. By knowing where, when, and how hard bones and teeth came about, we will be in a position to understand why. Why did our kind of hard tissues arise? Did they come about to protect animals from their environment? Did they come about to help them move? Answers to these questions lie in the fossil record, in rocks approximately 500 million years old.
Some of the most common fossils in ancient oceans, 500 million to 250 million years old, are conodonts. Conodonts were discovered in the 1830s by the Russian biologist Christian Pander, who will reappear in a few chapters. They are small shelly organisms with a series of spikes projecting out of them. Since Pander’s time, conodonts have been discovered on every continent; there are places where you cannot crack a rock without finding vast numbers of them. Hundreds of kinds of conodonts are known.
For a long time, conodonts were enigmas: scientists disagreed over whether they were animal, vegetable, or mineral. Everybody seemed to have a pet theory. Conodonts were claimed to be pieces of clams, sponges, vertebrates, even worms. The speculation ended when whole animals started to show up in the fossil record.
The first specimen that made sense of everything was found by a professor of paleontology rummaging through the basement at the University of Edinburgh: there was a slab of rock with what looked like a lamprey in it. You might recall lampreys from biology class—these are very primitive fish that have no jaws. They make their living by attaching to other fish and feeding on their bodily fluids. Embedded in the front of the lamprey impression were small fossils that looked strangely familiar. Conodonts. Other lamprey-like fossils started to come out of rocks in South Africa and later the western United States. These creatures all had an exceptional trait: they had whole assemblages of conodonts in their mouths. The conclusion became abundantly clear: conodonts were teeth. And not just any teeth. Conodonts were the teeth of an ancient jawless fish.
We had the earliest teeth in the fossil record for over 150 years before we realized what they were. The reason comes down to how fossils are preserved. The hard bits, for example teeth, tend to get preserved easily. Soft parts, such as muscle, skin, and guts, usually decay without fossilizing. We have museum cabinets full of fossil skeletons, shells, and teeth, but precious few guts and brains. On the rare occasions when we find evidence of soft tissues, they are typically preserved only as impressions or casts. Our fossil record is loaded with conodont teeth, but it took us 150 years to find the bodies. There is something else remarkable about the bodies to which conodonts belonged. They have no hard bones. These were soft-bodied animals with hard teeth.
For years, paleontologists have argued about why hard skeletons, those containing hydroxyapatite, arose in the first place. For those who believed that skeletons began with jaws, backbones, or body armor, conodonts provide an “inconvenient tooth,” if you will. The first hard hydroxyapatite-containing body parts were teeth. Hard bones arose not to protect animals, but to eat them. With this, the fish-eat-fish world really began in earnest. First, big fish ate little fish; then, an arms race began. Little fish developed armor, big fish obtained bigger jaws to crack the armor, and so on. Teeth and bones really changed the competitive landscape.
Things get more interesting still as we look at some of the first animals with bony heads. As we move up in time from the earliest conodont animals, we see what the first bony-head skeletons looked like. They belonged to fish called ostracoderms, are about 500 million years old, and are found in rocks all over the world, from the Arctic to Bolivia. These fish look like hamburgers with fleshy tails.
The head region of an ostracoderm is a big disk covered by a shield of bone, looking almost like armor. If I were to open a museum drawer and show you one, you would immediately notice something odd: the head skeleton is really shiny, much like our teeth or the scales of a fish.
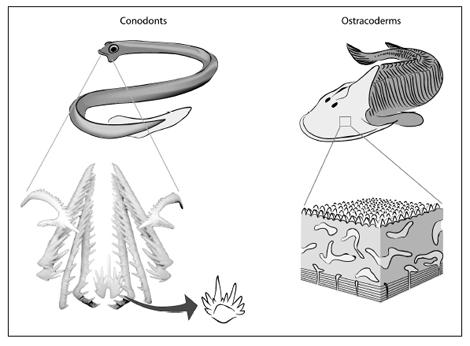
A conodont (left) and an ostracoderm (right). Conodonts were originally found isolated. Then, as whole animals became known, we learned that many of them functioned together as a tooth row in the mouths of these soft-bodied jawless fish. Ostracoderms have heads covered with a bony shield. The microscopic layers of that shield look like they are composed of little tooth-like structures. Conodont tooth row reconstruction courtesy of Dr. Mark Purnell, University of Leicester, and Dr. Philip Donoghue, University of Bristol.
One of the joys of being a scientist is that the natural world has the power to amaze and surprise. Here, in ostracoderms, an obscure group of ancient jawless fish, lies a prime example. Ostracoderms are among the earliest creatures with bony heads. Cut the bone of the skull open, embed it in plastic, pop it under the microscope, and you do not find just any old tissue structure; rather, you find virtually the same structure as in our teeth. There is a layer of enamel and even a layer of pulp. The whole shield is made up of thousands of small teeth fused together. This bony skull—one of the earliest in the fossil record—is made entirely of little teeth. Teeth originally arose to bite creatures; later, a version of teeth was used in a new way to protect them.
TEETH, GLANDS, AND FEATHERS

Teeth not only herald a whole new way of living, they reveal the origin of a whole new way of making organs. Teeth develop by an interaction of two layers of tissue in our developing skin. Basically, two layers approach each other, cells divide, and the layers change shape and make proteins. The outer layer spits out the molecular precursors of enamel, the inner layer the dentine and pulp of the inside of the tooth. Over time, the structure of the tooth is laid down, then tweaked to make the patterns of cusps and troughs that distinguish each species.
The key to tooth development is that an interaction between these two layers of tissue, an outer sheet of cells and an inner loose layer of cells, causes the tissue to fold and makes both layers secrete the molecules that build the organ. It turns out that exactly the same process underlies the development of all the structures that develop within skin: scales, hair, feathers, sweat glands, even mammary glands. In each case, two layers come together, fold, and secrete proteins. Indeed, the batteries of the major genetic switches that are active in this process in each kind of tissue are largely similar.
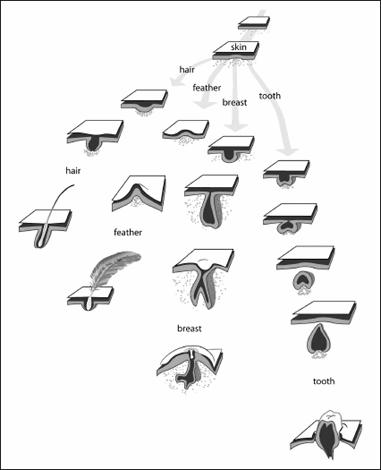
Teeth, breasts, feathers, and hair all develop from the interactions between layers of skin.
This example is akin to making a new factory or assembly process. Once plastic injection was invented, it was used in making everything from car parts to yo-yos. Teeth are no different. Once the process that makes teeth came into being, it was modified to make the diverse kinds of organs that lie within skin. We saw this taken to a very great extreme in the ostracoderms. Birds, reptiles, and humans are just as extreme in many ways. We would never have scales, feathers, or breasts if we didn’t have teeth in the first place. The developmental tools that make teeth have been repurposed to make other important skin structures. In a very real sense organs as different as teeth, feathers, and breasts are inextricably linked by history.
A theme of these first four chapters is how we can trace the same organ in different creatures. In Chapter 1 we saw that we can make predictions and find versions of our organs in ancient rocks. In Chapter 2 we saw how we can trace similar bones all the way from fish to humans. Chapter 3 shows how the real heritable part of our bodies—the DNA and genetic recipe that builds organs—can be followed in very different creatures. Here, in teeth, mammary glands, and feathers, we find a similar theme. The biological processes that make these different organs are versions of the same thing. When you see these deep similarities among different organs and bodies, you begin to recognize that the diverse inhabitants of our world are just variations on a theme.
CHAPTER FIVE

GETTING AHEAD
I
t was two nights before my anatomy final and I was in the lab at around two in the morning, memorizing the cranial nerves. There are twelve cranial nerves, each branching to take bizarre twists and turns through the inside of the skull. To study them, we bisected the skull from forehead to chin and sawed open some of the bones of the cheek. So there I was, holding half of the head in each hand, tracing the twisted paths that the nerves take from our brains to the different muscles and sense organs inside.
I was enraptured by two of the cranial nerves, the trigeminal and the facial. Their complicated pattern boiled down to something so simple, so outrageously easy that I saw the human head in a new way. That insight came from understanding the far simpler state of affairs in sharks. The elegance of my realization—though not its novelty; comparative anatomists had had it a century or more ago—and the pressure of the upcoming exam led me to forget where I was. At some point, I looked around. It was the middle of the night and I was alone in the lab. I also happened to be surrounded by the bodies of twenty-five human beings under sheets. For the first and last time, I got the willies. I worked myself into such a lather that the hairs on the back of my neck rose, my feet did their job, and within a nanosecond I found myself at the bus stop, out of breath. It goes without saying that I felt ridiculous. I remember telling myself: Shubin, you’ve become hard-core. That thought did not last long; I soon discovered I had locked my house keys in the lab.