Why Evolution Is True (12 page)
Read Why Evolution Is True Online
Authors: Jerry A. Coyne

We humans have many vestigial features proving that we evolved. The most famous is the appendix. Known medically as the vermiform (“worm-shaped”) appendix, it’s a thin, pencil-sized cylinder of tissue that forms the end of the pouch, or caecum, that sits at the junction of our large and small intestines. Like many vestigial features, its size and degree of development are highly variable: in humans, its length ranges from about an inch to over a foot. A few people are even born without one.
In herbivorous animals like koalas, rabbits, and kangaroos, the caecum and its appendix tip are much larger than ours. This is also true of leaf-eating primates like lemurs, lorises, and spider monkeys. The enlarged pouch serves as a fermenting vessel (like the “extra stomachs” of cows), containing bacteria that help the animal break down cellulose into usable sugars. In primates whose diet includes fewer leaves, like orangutans and macaques, the caecum and appendix are reduced. In humans, who don’t eat leaves and can’t digest cellulose, the appendix is nearly gone. Obviously the less herbivorous the animal, the smaller the caecum and appendix. In other words, our appendix is simply the remnant of an organ that was critically important to our leaf-eating ancestors, but of no real value to us.
Does an appendix do us any good at all? If so, it’s not obvious. Removing it doesn’t produce any bad side effects or increase mortality (in fact, removal seems to
reduce
the incidence of colitis). Discussing the appendix in his famous textbook
The Vertebrate Body
, the paleontologist Alfred Romer remarked dryly, “Its major importance would appear to be financial support of the surgical profession.” But to be fair, it may be of some small use. The appendix contains patches of tissue that may function as part of the immune system. It has also been suggested that it provides a refuge for useful gut bacteria when an infection removes them from the rest of our digestive system.
reduce
the incidence of colitis). Discussing the appendix in his famous textbook
The Vertebrate Body
, the paleontologist Alfred Romer remarked dryly, “Its major importance would appear to be financial support of the surgical profession.” But to be fair, it may be of some small use. The appendix contains patches of tissue that may function as part of the immune system. It has also been suggested that it provides a refuge for useful gut bacteria when an infection removes them from the rest of our digestive system.
But these minor benefits are surely outweighed by the severe problems that come with the human appendix. Its narrowness makes it easily clogged, which can lead to its infection and inflammation, otherwise known as appendicitis. If not treated, a ruptured appendix can kill you. You have about one chance in fifteen of getting appendicitis in your lifetime. Fortunately, thanks to the evolutionarily recent practice of surgery, the chance of dying when you get appendicitis is only 1 percent. But before doctors began to remove inflamed appendixes in the late nineteenth century, mortality may have exceeded 20 percent. In other words, before the days of surgical removal, more than one person in a hundred died of appendicitis. That’s pretty strong natural selection.
Over the vast period of human evolution—more than 99 percent of it—there were no surgeons, and we lived with a ticking time bomb in our gut. When you weigh the tiny advantages of an appendix against its huge disadvantages, it’s clear that on the whole it is simply a bad thing to have. But apart from whether it’s good or bad, the appendix is still vestigial, for it no longer performs the function for which it evolved.
So why do we still have one? We don’t yet know the answer. It may in fact have been on its way out, but surgery has almost eliminated natural selection against people with appendixes. Another possibility is that selection simply can’t shrink the appendix any more without it becoming even
more
harmful: a smaller appendix may run an even higher risk of being blocked. That might be an evolutionary roadblock to its complete disappearance.
more
harmful: a smaller appendix may run an even higher risk of being blocked. That might be an evolutionary roadblock to its complete disappearance.
Our bodies teem with other remnants of primate ancestry. We have a vestigial tail: the coccyx, or the triangular end of our spine that’s made of several fused vertebrae hanging below our pelvis. It’s what remains of the long, useful tail of our ancestors (figure 14). It still has a function (some useful muscles attach to it), but remember that its vestigiality is diagnosed not by its usefulness but because it no longer has the function for which it originally evolved. Tellingly, some humans have a rudimentary tail muscle (the “extensor coccygis”), identical to the one that moves the tails of monkeys and other mammals. It still attaches to our coccyx, but since the bones can’t move, the muscle is useless. You may have one and not even know it.
Other vestigial muscles become apparent in winter, or at horror movies. These are the
arrector pili,
the tiny muscles that attach to the base of each body hair. When they contract, the hairs stand up, giving us “goose bumps”—so called because of their resemblance to the skin of a plucked goose. Goose bumps and the muscles that make them serve no useful function, at least in humans. In other mammals, however, they raise the fur for insulation when it’s cold, and cause the animal to look larger when it’s making or receiving threats. Think of a cat, whose fur bushes out when it’s cold or angry. Our vestigial goose bumps are produced by exactly the same stimuli—cold or a rush of adrenaline.
arrector pili,
the tiny muscles that attach to the base of each body hair. When they contract, the hairs stand up, giving us “goose bumps”—so called because of their resemblance to the skin of a plucked goose. Goose bumps and the muscles that make them serve no useful function, at least in humans. In other mammals, however, they raise the fur for insulation when it’s cold, and cause the animal to look larger when it’s making or receiving threats. Think of a cat, whose fur bushes out when it’s cold or angry. Our vestigial goose bumps are produced by exactly the same stimuli—cold or a rush of adrenaline.
And here’s a final example: if you can wiggle your ears, you’re demonstrating evolution. We have three muscles under our scalp that attach to our ears. In most individuals they’re useless, but some people can use them to wiggle their ears. (I am one of the lucky ones, and every year demonstrate this prowess to my evolution class, much to the students’ amusement.) These are the same muscles used by other animals, like cats and horses, to move their ears around, helping them localize sounds. In those species, moving the ears helps them detect predators, locate their young, and so on. But in humans the muscles are good only for entertainment.
16
16
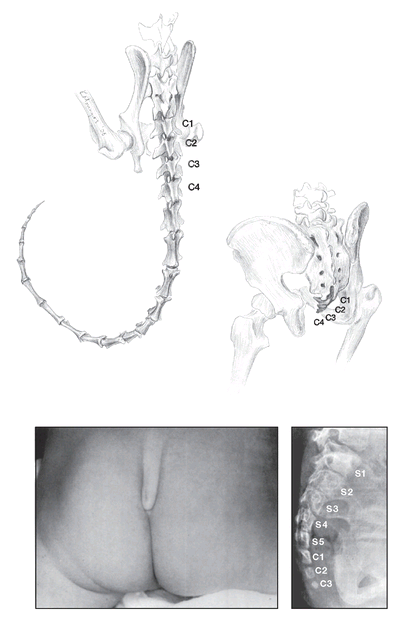
FIGURE 14
. Vestigial and atavistic tails. Top left: in our relatives that have tails, such as the ruffed lemur
(Varecia variegates),
the tail (caudal) vertebrae are unfused (the first four are labeled C1-C4). But in the human “tail,” or coccyx (top right), the caudal vertebrae are fused to form a vestigial structure. Bottom: atavistic tail of a three-month-old Israeli infant. X-ray of the tail (right) shows that the three caudal vertebrae are much larger and more well developed than normal, are not fused, and approach the size of the sacral vertebrae (S1-S5). The tail was later surgically removed.
. Vestigial and atavistic tails. Top left: in our relatives that have tails, such as the ruffed lemur
(Varecia variegates),
the tail (caudal) vertebrae are unfused (the first four are labeled C1-C4). But in the human “tail,” or coccyx (top right), the caudal vertebrae are fused to form a vestigial structure. Bottom: atavistic tail of a three-month-old Israeli infant. X-ray of the tail (right) shows that the three caudal vertebrae are much larger and more well developed than normal, are not fused, and approach the size of the sacral vertebrae (S1-S5). The tail was later surgically removed.
To paraphrase the quote from the geneticist Theodosius Dobzhanslcy that begins this chapter, vestigial traits make sense only in the light of evolution. Sometimes useful, but often not, they’re exactly what we’d expect to find if natural selection gradually eliminated useless features or refashioned them into new, more adaptive ones. Tiny, nonfunctional wings, a dangerous appendix, eyes that can’t see, and silly ear muscles simply don’t make sense if you think that species were specially created.
AtavismsOCCASIONALLY AN INDIVIDUAL crops up with an anomaly that looks like the reappearance of an ancestral trait. A horse can be born with extra toes, a human baby with a tail. These sporadically expressed remnants of ancestral features are called
atavisms,
from the Latin
atavus,
or “ancestor:” They differ from vestigial traits because they occur only occasionally rather than in every individual.
atavisms,
from the Latin
atavus,
or “ancestor:” They differ from vestigial traits because they occur only occasionally rather than in every individual.
True atavisms must recapitulate an ancestral trait, and in a fairly exact way. They aren’t simply monstrosities. A human born with an extra leg, for example, is not an atavism because none of our ancestors had five limbs. The most famous genuine atavisms are probably the legs of whales. We’ve already learned that some species of whales retain vestigial pelvises and rear leg bones, but about one whale in five hundred is actually born with a rear leg that protrudes outside the body wall. These limbs show all degrees of refinement, with many of them clearly containing the major leg bones of terrestrial mammals-the femur, tibia, and fibula. Some even have feet and toes!
Why do atavisms like this occur at all? Our best hypothesis is that they come from the reexpression of genes that were functional in ancestors but were silenced by natural selection when they were no longer needed. Yet these dormant genes can sometimes be reawakened when something goes awry in development. Whales still contain some genetic information for making legs—not perfect legs, since the information has degraded during the millions of years that it resided unused in the genome—but legs nonetheless. And that information is there because whales descended from fourlegged ancestors. Like the ubiquitous whale pelvis, the rare whale leg is evidence for evolution.
Modern horses, which descend from smaller, five-toed ancestors, show similar atavisms. The fossil record documents the gradual loss of toes over time, so that in modern horses only the middle one—the hoof—remains. It turns out that horse embryos begin development with three toes, which grow at equal rates. Later, however, the middle toe begins to grow faster than the other two, which at birth are left as thin “splint bones” along either side of the leg. (Splint bones are true vestigial features. When they become inflamed, a horse gets “the splints.”) On rare occasions, though, the extra digits continue developing until they become true extra toes, complete with hoofs. Often these atavistic toes don’t touch the ground unless the horse is running. This is exactly what the ancient horse
Merychippus
looked like 15 million years ago. Extra-toed horses were once considered supernatural wonders: both Julius Caesar and Alexander the Great were said to have ridden them. And they are wonders of a sort—wonders of evolution—for they clearly show genetic kinship between ancient and modern horses.
Merychippus
looked like 15 million years ago. Extra-toed horses were once considered supernatural wonders: both Julius Caesar and Alexander the Great were said to have ridden them. And they are wonders of a sort—wonders of evolution—for they clearly show genetic kinship between ancient and modern horses.
The most striking atavism in our own species is called the “coccygeal projection,” better known as the human tail. As we’ll learn shortly, early in development human embryos have a sizable fishlike tail, which begins to disappear about seven weeks into development (its bones and tissues are simply reabsorbed by the body). Rarely, however, it doesn’t regress completely, and a baby is born with a tail projecting from the base of its spine (figure 14). The tails vary tremendously: some are “soft,” without bone, while others contain vertebrae—the same vertebrae normally fused together in our tailbone. Some tails are an inch long, others nearly a foot. And they aren’t just simple flaps of sltin, but can have hair, muscles, blood vessels, and nerves. Some can even wiggle! Fortunately, these awkward protrusions are easily removed by surgeons.
What could this mean, other than that we still carry a developmental program for making tails? Indeed, recent genetic work has shown that we carry exactly the same genes that make tails in animals like mice, but these genes are normally deactivated in human fetuses. Tails appear to be true atavisms.
Some atavisms can be produced in the laboratory. The most amazing of these is that paragon of rarity, hen’s teeth. In 1980, E. J. Kollar and C. Fisher at the University of Connecticut combined the tissues of two species, grafting the tissue lining the mouth of a chicken embryo on top of tissue from the jaw of a developing mouse. Amazingly, the chicken tissue eventually produced toothlike structures, some with distinct roots and crowns. Since the underlying mouse tissue alone could not produce teeth, Kollar and Fisher inferred that molecules from the mouse reawakened a dormant developmental program for making teeth in chickens. This meant that chickens had all the right genes for making teeth, but were missing a spark that the mouse tissue was able to provide. Twenty years later, scientists unraveled the molecular biology and showed that Kollar and Fisher’s suggestion was right: birds do indeed have genetic pathways for producing teeth, but don’t make them because a single crucial protein is missing. When that protein is supplied, toothlike structures form on the bill. You’ll remember that birds evolved from toothed reptiles. They lost those teeth more than 60 million years ago, but clearly still carry some genes for making them—genes that are remnants of their reptilian ancestry.
Dead GenesATAVISMS AND VESTIGIAL TRAITS show us that when a trait is no longer used, or becomes reduced, the genes that make it don’t instantly disappear from the genome: evolution stops their action by inactivating them, not snipping them out of the DNA. From this we can make a prediction. We expect to find, in the genomes of many species, silenced, or “dead,” genes: genes that once were useful but are no longer intact or expressed. In other words, there should be vestigial genes. In contrast, the idea that all species were created from scratch predicts that no such genes would exist, since there would be no common ancestors in which those genes were active.
Thirty years ago we couldn’t test this prediction because we had no way to read the DNA code. Now, however, it’s quite easy to sequence the complete genome of species, and it’s been done for many of them, including humans. This gives us a unique tool to study evolution when we realize that the normal function of a gene is to make a protein—a protein whose sequence of amino acids is determined by the sequence of nucleotide bases that make up the DNA. And once we have the DNA sequence of a given gene, we can usually tell if it is expressed normally—that is, whether it makes a functional protein—or whether it is silenced and makes nothing. We can see, for example, whether mutations have changed the gene so that a usable protein can no longer be made, or whether the “control” regions responsible for turning on a gene have been inactivated. A gene that doesn’t function is called a
pseudogene.
pseudogene.
Other books
Blind Fury by Linda I. Shands
The Widow and the Wastrel by Janet Dailey
To Each Her Own (The Swirl Book 1) by Sylvia Sinclair
Snowjob by Ted Wood
October Fest: A Murder-by-Month Mystery by Jess Lourey
Firecracker Gone Astray - Firecracker #2 (Erotic Romance) by Flint, Megan
She's Out by La Plante, Lynda
Into the Fire: A Firsthand Account of the Most Extraordinary Battle in the Afghan War by West, Bing, Meyer, Dakota
Dust: Before and After by S.E. Smith
Playing Dirty by Kiki Swinson