Whole (22 page)
Authors: T. Colin Campbell

As a research discipline, modern-day genetics addresses the consequences of that small percentage of disease-producing genes that we are born with in addition to those damaged genes that we acquire along the way. It operates from the assumption that one day we will be able to locate and identify damaged genes and use that information to more easily diagnose and treat disease. However, it largely fails to consider how to prevent genes from becoming damaged in the first place. And the field’s presumption that genetic engineering will be able to prevent disease from occurring by repairing or replacing specific genes that cause disease, is the height of hubris, given the unimaginable complexity of DNA.
The explanatory model long used by cancer researchers postulates that cancer begins either with an inherited gene or with a gene that has been damaged by a carcinogen or other factor during a person’s lifetime, with different cancer types having different genetic starting points. If the damaged gene or genes are not repaired or removed, the damage will become a permanent part of the cell’s genetic code, passed on to each successive generation of cells. This series of cell generations grow into cell masses, then tumor masses, theoretically at a somewhat faster or uninhibited rate. The presumption here is that this process is fixed, with virtually no opportunities for its reversal. If the cell and damaged gene replicate, there is nothing that can be done; the result is cancer. More damaged genes mean more cancer; fewer damaged genes mean less cancer (see
Figure 9-1
).
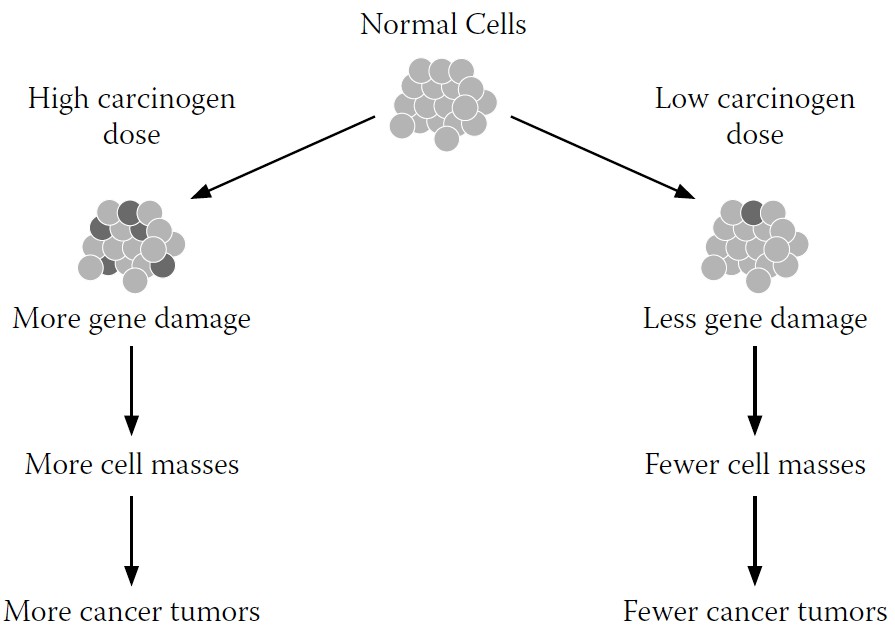
FIGURE 9-1.
Traditional explanatory model for cancer development
However, research has shown that there are other environmental factors involved in whether damaged DNA becomes cancer. During my laboratory work with AF, one line of research showed that even when we had genetically predisposed a mouse or rat to develop liver cancer by
intentionally damaging its genes through exposure to hepatitis B or to a high dose of AF, the cancer would develop
only in the presence of a high
-
animal-protein diet.
In other words, nutrition trumped environment, even when the environment was particularly nasty. Although their DNA had been damaged, cancer did not inevitably result (see
Figure 9-2
).
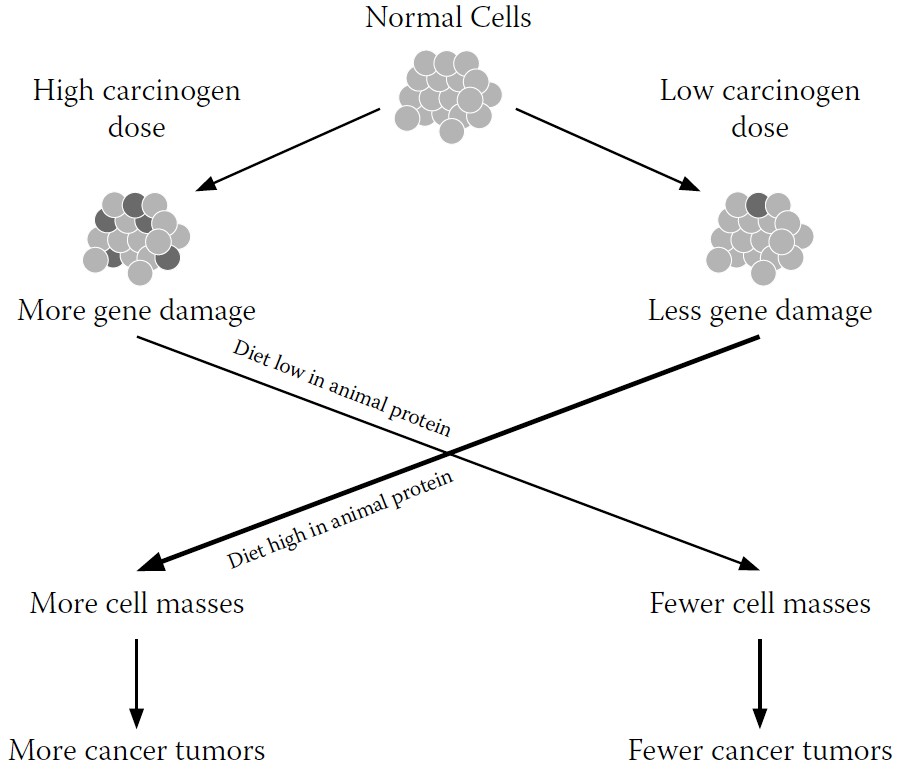
FIGURE 9-2.
Revised explanatory model for cancer development
There’s also evidence from human subjects, which you can read in depth in
The China Study,
that supports the idea that the foods we eat and the nutrition they provide is far more important in determining cancer than our genetic backgrounds.
1
Population studies begun forty to fifty years ago show that when people migrate from one country to another, they acquire the cancer rate of the country to which they move, despite the fact their genes remain the same. This strongly indicates that at least 80 percent to 90 percent—and probably closer to 97 percent to 98 percent—of all cancers are related to diet and lifestyle, not to genes.
Also, comparisons of cancer rates among identical twins show that even though both members of a twin pair have the same DNA, most of the time they fail to get the same cancers. If genes alone were sufficient for cancer development, you’d expect them to get the same cancer nearly 100 percent of the time. (For those relatively few twins who do get the same cancer, their dietary similarities could be at least partly responsible.)
In short, proper nutrition doesn’t just prevent damage; it affects the way our bodies respond to already damaged genes, often mitigating disease symptoms as they arise or even preventing them completely, sometimes with no additional medication or other treatments needed. In experimental animal studies in my own laboratory, cancer progression could even be reversed by nutritional changes. And researchers are now producing evidence that WFPB nutrition can turn cancer-producing genes off altogether.
All this suggests that the way cancer works is a far cry from the way cancer researchers assume it works—and of course, how something works has major implications for the way we go about fighting it.
The more work I did with AF and diet, the more I became convinced that AF wasn’t the villain most scientists assumed it to be when it came to liver cancer. In fact, I started to see that none of the accepted “causes” of cancer, in the absence of a high-animal-protein diet, mattered that much. Not genetics, not chemical carcinogens like AF, not viruses. But the cancer industry, researchers, policy makers, the media, and the public focus almost exclusively on genes, chemicals, and viruses. Nutrition did not even make the list, even though it was becoming clear from my experiments and those of others that nutrition was cancer’s on-off switch.
Our offensive strategy in the War on Cancer primarily involves two main methods of prevention: controlling the expression of cancer-producing genes (by replacing or manipulating them), and getting rid of all environmental substances that might trigger genetic mutations. We saw in
chapter eight
why focusing on manipulating genes themselves will not be effective. But purging our environments of toxins isn’t the answer, either. First, it can’t be done. Even if we could remove all the human-made
toxins from our environment (an effort I wholeheartedly support), nature still provides us with many mutagenic phenomena that we can’t regulate or engineer out of existence, like sunlight and radon. Second, and more to the point, the effect of these environmental mutagens (substances that cause mutations in DNA) is mostly trumped by good nutrition. Yet these findings haven’t stopped the government from spending far more time and money chasing after environmental carcinogens that are supposedly causing cancer by creating gene mutations than on promoting WFPB nutrition.
You can’t turn around without hearing about another potential source of cancer to avoid: toxic chemicals, viruses, cell phones, the sun... A recent
New York Times
article titled, “Is It Safe to Play Yet?” chronicles the almost paralyzing fears expressed by young parents trying to give their children a healthy start. Many of them purge their homes of makeup, shampoos, detergents, plastic cups and bottles, laminated furniture, and even rubber duckies.
2
And every so often the media will gravitate toward a terrifying story of a cancer-causing agent in our midst. Alar, a common pesticide used on apples. Microwave ovens. Power lines near homes. Enormous public concern often arises. Then, adding fuel to the fire, we are reminded that an ever-increasing number of chemicals—some intentional, some not— are being added to our personal and public environments (food, water, cosmetics). And finally, we are told that only a tiny fraction (perhaps 2,000) of these chemicals (about 80,000 or so) have been tested for their carcinogenicity.
Social activists speak out, and rightly so, against “cancer clusters”: areas where there are abnormally high rates of particular cancers, presumably due to toxic dumping and other nasty practices that befall low-income communities but not their wealthier neighbors. Communities battle each other in NIMBY (not in my backyard) skirmishes that aim to move the toxic output as far away as possible. Movies like
Erin Brockovich
and
A Civil Action
convince us to buy bottled water or install kitchen filters to keep contaminants out of our homes.
The result of this constant onslaught is a pervasive sense of fear that either morphs into passivity (“I give up, there’s nothing I can do”) or obsessive action (“Let’s live in a bubble”). Ultimately, however, neither does much to reduce our cancer risk.
I’m not saying we shouldn’t work to block new onslaughts of toxicity. I should know; my speech suffered for decades from my exposure to dioxin, one of the most toxic chemicals known to humans, and one I helped discover when, as a postdoctoral researcher at MIT in the 1960s, I isolated it from oil used in poultry feed.
3
As individuals, we should seek to minimize our exposure to carcinogens. And as a society, we should err on the side of over-caution before approving and disseminating new technologies and substances into our water, air, and soil.
But carcinogenic testing has become a self-perpetuating industry rather than a safeguard of public health. From its origins shortly after the discovery in the 1950s of a harmful chemical agent in a spray used on cranberries, this program has grown to a hundred-million-dollar program today. It is difficult to estimate the total costs for this program because of its secondary effects on regulatory and cancer control programs, but, in my estimation, it surely has amounted in total to tens of billions of wasted dollars. And although the goal of reducing environmental toxins is laudable, the government’s approach to this is ineffective and misleading.
The chief arm of the U.S. government’s war on “stuff that may cause cancer”—and the poster child for how our current approach wastes time and money—is the carcinogen bioassay program, a multimillion-dollar program that researches hundreds of chemicals in an attempt to figure out which ones cause cancer in humans.
In 1958, the U.S. government added a clause to the Food Additive Amendment of the Food and Drug Act that specified that no chemical should be added to our food supply if it was found to be carcinogenic. One natural outgrowth of the clause was that the government needed a way to determine which chemicals were, in fact, carcinogenic. So a program was set up to do just that. Known popularly as the carcinogen bioassay program (CBP), it seems at first blush like a very good thing: figure out what’s harmful and keep it out of our food supply.
The problem is, the reductionist assumptions that underpin the program, from the idea that environmental toxins inevitably lead to cancer, to the ill-considered design of the program’s research and testing methods,
call its usefulness into question. The CBP distracts us from the significant and easily addressed causes of cancer, and directs us to secondary factors over which we have almost no control, thus accomplishing little and diverting resources from initiatives that could make a significant difference.
The CBP tests the ability of suspect chemicals to cause cancer in experimental animals (rats and mice) within their lifetimes (about two years). If enough of the lab animals get cancer while being dosed with a particular chemical, it is labeled a carcinogen. If supporting evidence shows a statistically significant (albeit usually contested) association with humans, it is labeled a human carcinogen. Some examples of human carcinogens identified by the CBP include dioxin, formaldehyde, asbestos, DDT (insecticide spray), polycyclic aromatic hydrocarbons (PAHs, in smoked foods and cigarettes), nitrosamines (in bacon and hot dogs), PCBs (used in the manufacture of electrical transformers), benzene (found in solvents, gasoline, and cigarette smoke), and of course the subject of my lab’s work, AF.
When the CBP selects a chemical for cancer risk evaluation, it starts with animal trials. First, the researchers select the animal (rat or mouse). Next, the rodents are dosed with levels of the suspected carcinogen about a thousand to ten thousand times higher than the equivalent doses that humans are expected to encounter. If a significant percentage of the animals develop cancer, the substance is classified as a carcinogen.
You may have noticed two gaping holes in this logic. First, there’s the assumption that if very high doses of a chemical cause cancer, then much lower doses must also cause cancer. Maybe not as often or as lethally, and maybe not as quickly, but cancer is still assumed to be the end result. In science-speak, this assumption is known as “high-dose to low-dose interpolation.” This is a very uncertain procedure because we don’t really know if the straight-line relationship seen at these exceptionally high doses continues to be linear all the way down to the much lower doses typically observed for human exposure. What if the high dose is like getting hit by a car, while the low dose is like getting hit by a Matchbox car? The high dose of the nonnutritive sweetener saccharin that caused a very
small increase in bladder cancer in laboratory rats was equivalent to the human consumption of 1,200 cans of diet soda in a day. Silly? I think so. And it should be added, as already discussed, that the body is capable of repairing much of the damage that low levels of natural chemicals cause.