The Giza Power Plant (29 page)

- Was there just one of each item?
- Were they found together in the same shaft?
- Were they in any way connected?
- Was their removal from the shaft difficult or easy?
If we work on the premise that the items were discovered in the same shaft and were connected in some way, or were able to be joined together, we could theorize that they might have been part of the switching mechanism that signaled the need for more chemicals. This hypothesis makes sense when viewed in context with other evidence from the Queen's Chamber. When we review the details researchers have noted inside the Queen's Chamber, all these seemingly unexplained facts fall into place, if our explanation is founded on the basic premise that some kind of chemical reaction was taking place there.
There is a corbelled niche with a small tunnel cut to a depth of thirty-eight feet, ending in a bulb-shaped cavern. Hydraulics engineer Edward Kunkle (of Ohio and now deceased) questioned the official explanation that this tunnel was cut by treasure seekers and claimed instead that with its flat, level floor and its almost perfect right-angled left side, it must have been part of the original construction. What is more, in Kunkle's view its features show it could have served a mechanical purpose. Kunkle proposed that it was part of a large ram pump, which also involved other features located inside the Great Pyramid.
In his book
5/5/2000
Ice: The Ultimate Disaster,
Richard Noone focused upon the mysteries of this chamber and asked a tantalizing question. Noone went further in his search for reasons for the presence of salt in this chamber. He interviewed Dr. William Tiller of Stanford University, whose research deals with the science of crystallization, surfaces, and interphases between two media, a science concerning biomaterials and psychoenergetics. Other than an interesting comment on "energy resonances" that he says would be particularly good to excite things in a pyramid, Tiller could not shed any light on the cause of the mysterious salt encrustations. Noone, however, had an enlightening dialogue with him:
Noone:
Dr. Tiller, why would the Middle Chamber of the Great Pyramid be such a magnet, or repository for these crystals?
Tiller:
That's really tough to imagine, because you basically have got to have (a) either some salt water which was in there and evaporated and then deposited this because it got very hot, or (b) it was at some time under the ocean and the water seeped in, or (c) somehow the "energies" converted what was in the walls to sodium chloride. None of these, however, makes any sense at all to me. I regret I don't have, at this point in any event, a really good answer for
you.
3
I was hoping to be able to get into the Queen's Chamber while I was in Egypt in 1986 to get a sample of the salt for analysis. I had speculated that the salt on the walls of the chamber was an unwanted, though significant, residual substance caused by a chemical reaction where hot hydrogen reacted with the limestone. Unfortunately, I was unable to get into the chamber because a French team was already inside the Horizontal Passage, boring holes into what they hoped were additional chambers. (It was discovered, after I left Egypt, that the spaces contained only sand.)
As it turned out, my research would have been redundant. Noone reported in his book that another individual had already had the same idea and done the work. In 1978, Dr. Patrick Flanagan asked the Arizona Bureau of Geology and Mineral Technology to analyze a sample of this salt. They found it to be a mixture of calcium carbonate (limestone), sodium chloride (halite or salt), and calcium sulfate (gypsum, also known as plaster of paris). These are precisely the minerals that would be produced by the reaction of hot, hydrogen-bearing gas with the limestone walls and ceiling of the Queen's Chamber.
Armed with this information, I sought out a chemical engineer, Joseph Drejewski, to see if my perception regarding the Queen's Chamber was plausible. He was skeptical about my entire premise but agreed to look at the data and form an evaluation.
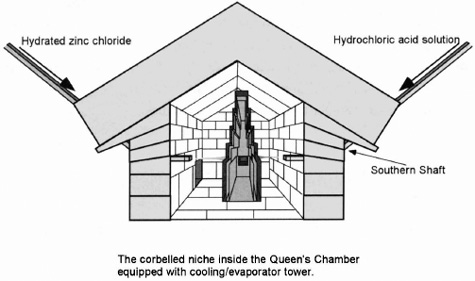
I had speculated that the five-inch "left"âwhich contained a small hole through to the channelâthat prevented each shaft from joining with the Queen's Chamber was intentionally designed to meter a specific amount of fluid into the chamber over a period of time. If we knew the head pressure of the fluid, we could accurately calculate the amount of fluid that flowed through this "left". One of these two shafts has a different discoloration,
or staining, and I speculated that this was the result of the ancient Egyptians introducing two different chemicals into the chamber, which, when combined, would produce a reaction. Drejewski agreed that two chemical solutions could be introduced into this chamber to create hydrogen or ammonia under ambient conditions of 80° Fahrenheit, ± 20°. He agreed that the niche in the wall of the chamber could have been used to house a cooling or evaporation tower. The corbelled niche inside the reaction chamber would have provided an anchor for this tower, which also may have contained a catalyst (see Figure 61). One scenario could be that the chemicals pooled on the floor of the chamber and wicked through the catalyst material. The offset of the niche may indicate the proportion of each chemical introduced into the chamber. Drejewski, therefore, agreed that my theory was plausible.
To evaluate my theory further, we must now move from considering the technology of this machine to the fuel that ran it. Let us consider how hydrogen is made and used to produce energy (see Figure 62). Drejewski prepared a report informing me of the following:
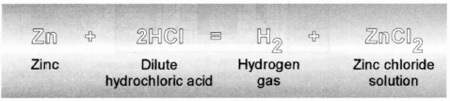
Hydrogen is most easily obtained by displacing it from acids by contact
with certain metals that are more active than hydrogen and therefore will combine more readily with the other constituents of the acid. Zinc (Zn) is the most commonly chosen metal and when treated with dilute hydrochloric acid (HCl), it will produce a reasonably pure hydrogen gas which evolves at a relatively fast rate. The hydrogen gas produced by this reaction of zinc with hydrochloric acid may contain water vapor carried along by the gas as it bubbles through the water solution. If impurities are present, it is possible to remove the water vapor (with the impurity) bypassing the generated gas over or through a drying agent such as calcium chloride (Ca Cl
2
), which retains the water vapor, but does not react with the hydrogen gas. Other metals which can be used [as a drying agent] are magnesium and finely divided iron
(powder).
4
F
IGURE
61.
Cooling/Evaporator Tower
Drejewski ended his evaluation by cautiously stating, "It is highly probable that [through this reaction] impurities such as calcium sulfate (gypsum) and sodium chloride (halite) can be leached through limestone [calcium carbonate] (Ca CO
3
)."
I have been asked by other researchers who have reviewed a synopsis of my theory whether electrolysis could have played a part in the generation of hydrogen. I am not going to rule that out completely, but electrolysis would require only one shaft leading to the chamber, as it is a process using only water and electrical power. We have to explain the reasons for two similar shafts, and the dark staining inside the Northern Shaft. This staining dearly indicates the use of two different chemicals.
Additional evidence to support the theory that chemicals were flowing
down these shafts came in 1993, when Rudolph Gantenbrink guided his robot Upuaut II up the Southern Shaft and discovered the so-called "door" with its copper fittings. We will remember that Smyth noted gypsum exuding from the joints of the Southern Shaft leading to the Queen's Chamber. The filming of this channel by Gantenbrink's robot revealed signs of erosion in the lower portion of the shaft. The walls and floor of the channel were extremely rough, and the erosion of the walls appeared to have horizontal striations. There also were signs of what appeared to be gypsum leaching from the limestone walls. Having reached an opinion regarding the function of the Queen's Chamber in the Great Pyramid, I was quite intrigued when the discoveries in this channel were publicized. I did not know if my speculations would be bolstered by what was found there or if I would have to throw them out. As it happened, my theorized function of the Queen's Chamber within the Giza power plant was strengthened.
F
IGURE
62.
The Chemical Process to Produce Hydrogen
I have proposed that the Queen's Chamber was designed to allow the necessary chemical elements into the chamber at a metered rate. However, considering that the limestone masonry blocks inside the Queen's Chamber had perfectly fitted joints, we could reasonably ask if the crack in the wall was really an anomaly or if, instead, it was part of the original design. In the context of my theory, and if so designed, this "left" with the crack in it could have served to meter the chemicals into the chamber.
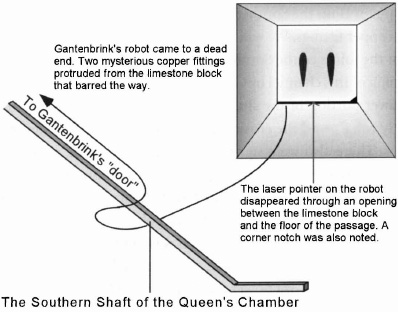
Gantenbrink's robot came to a dead end at the upper part of the Southern Shaft. It encountered a block of limestone with two mysterious copper fittings protruding through it. It was widely publicized that a hidden door had been found inside the Great Pyramid (see Figure 63). What was not publicized, however, is that the shaft itself is only about nine inches square. The so-called "door," I believe, is a misnomer. As for the copper fittingsâin the documentary they are presented as being stops to prevent the limestone block in which they are located from being raised. But this explanation does not fit. Why would the pyramid builders want to include a sliding block in an inaccessible area? And even if they did, how was it activated?
While I was watching the video of the exploration with my friend, Jeff Summers, he off-handedly remarked that the fittings looked like electrodes. His observation made sense to me. To deliver an accurate measure of hydrochloric acid solution to the reaction chamber, a certain head pressure would
need to be maintained. The head pressure is determined by the volume of fluid in the channel, that is, the weight of the column of chemical. The copper fittings would have served as a switch to signal the need for more chemicals. Floating on the surface of the fluid would have been another part of this switchâthe cedarlike wood joined together with the bronze grapnel hook. This assembly would rise and fall with the fluid in the channel. With the channel full, the bronze prongs would have made contact with the electrodes, creating a circuit, and as the fluid in the channel dropped, the prongs would move away from the electrodes, thereby breaking the contact and acting as a switch to signal the pumping of more chemical solution into the channel until the bronze hook again made contact with the electrodes (see Figure 64). As the rate of supply into the reaction chamber was slight, a small opening was all that was needed to maintain the supply of chemicals. The limestone door with its copper fittings, brought into the view of the camera when Upuaut II had gone as far as it could, has a slight gap at the bottom, under which the robot's laser light disappeared. There also is a notch in the bottom right-hand corner. All these features serve to support the speculation that the Egyptians were supplying a fluid to the Queen's Chamber
shafts, and it was necessary to maintain the fluid level in the shafts so that the weight of the fluid assured a constant and precise flow through the "left" in the chamber wall.