The Cosmic Landscape (28 page)

In the first minutes after the Big Bang, there were no atoms or even nuclei. Hot plasma composed of protons, neutrons, and electrons filled all space. The high temperature prevented nucleons from sticking together to form nuclei. As the universe cooled, protons and neutrons stuck together and formed the primordial elements.
4
But apart from tiny traces of other elements, only the very simplest of nuclei formed: hydrogen and helium.
Moreover, as medieval alchemists discovered, it’s not easy to transmute one element into another. So where, then, did all the carbon, oxygen, nitrogen, silicon, sulfur, iron, and other familiar chemical elements come from? The answer is that the intensely hot nuclear furnace of a star can do what no alchemists ever could—transform the elements, one into another. The cooking process is nuclear fusion, the same kind of fusion that powers nuclear weapons. Fusion combines the hydrogen nuclei in all sorts of permutations and combinations. The results of these nuclear reactions were the familiar elements.
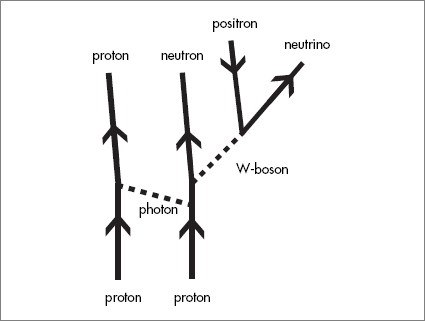
The chain of nuclear reactions in stars that starts with the lightest elements and leads to iron is complicated. A couple of examples will illustrate the point. The most familiar example is the fusion reaction that begins with hydrogen and produces helium. Here is where the weak interactions (diagrams with W- and Z-bosons) come in. The first step is the collision of two protons.
5
Many things can happen when two protons collide, but if you know the Feynman diagrams for the Standard Model, you can find one that ends up with a proton, a neutron, a positron, and a neutrino.
The positron finds a wandering electron in the star, and together they self-destruct into photons that eventually become the star’s thermal energy (heat). The neutrino just zips away and disappears with almost the speed of light. That leaves one sticky proton and one sticky neutron that stick together to form an isotope of hydrogen called deuterium.
Next, a third proton strikes the deuterium nucleus and sticks to it. The nucleus with two protons and a neutron is a form of helium called helium-three (
3
He), but it’s not the stable kind of helium that we use to fill balloons. That stuff is called helium-four (
4
He).
The story continues: two
3
He nuclei collide. All together that means four protons and two neutrons. But they don’t all stick together. Two of the protons fly off and leave a nucleus with two protons and two neutrons. That’s an ordinary
4
He nucleus. You don’t need to remember all that. Very few physicists do.
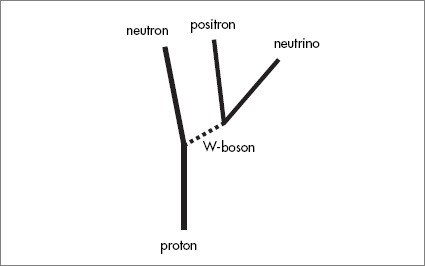
Most of the nuclear reactions that take place in stars consist of a single proton colliding with an already present nucleus and increasing its atomic weight by one unit. Sometimes the proton turns into a neutron by giving off a positron and a neutrino. Sometimes a neutron will become a proton, electron, and antineutrino. In any case, inside the star, step-by-step, the original hydrogen and helium nuclei turn into heavier elements.
But what good are the complex elements locked up inside stars? Science-fiction stories might posit strange forms of life made of swirling hot plasma that thrive at millions of degrees, but real life needs a cooler environment. Sadly, the carbon and oxygen remained imprisoned in the star’s interior throughout the entire lifetime of the star.
But stars don’t live forever.
Eventually all stars, our sun included, will run out of fuel. At that point a star collapses under its own weight. Before the fuel runs out, stars are kept in equilibrium by the heat and pressure generated by nuclear reactions. There are two competing tendencies in the star. Like a nuclear bomb, it wants to explode, while at the same time gravity is trying to crush it under its own enormous weight. These two tendencies, exploding and imploding, are kept in balance as long as there is fuel to burn. But once the fuel runs out, there is nothing to resist the pull of gravity, and the star implodes.
There are three possible endpoints to the implosion. A star like our sun is relatively light, and it will collapse only until it forms a white dwarf. A white dwarf is made of more or less ordinary material—protons, neutrons, and electrons—but the electrons are squeezed up against one another to a far greater degree than in ordinary materials. It’s the Pauli exclusion principle that keeps the electrons from collapsing even further. If all stars ended up as white dwarfs, the freshly cooked elements would remain imprisoned inside them.
On the other hand, if the star is many times heavier than the sun, the force of gravity will be irresistible. The inevitable disastrous collapse will end in the most violent process imaginable—the formation of a black hole. Elements trapped in black holes would be even less available than those in white dwarfs.
But there is a middle ground. Stars within a certain range of masses collapse past the white dwarf stage but not all the way to a black hole. In these stars the electrons, in a sense, get squeezed out, while the protons turn into neutrons, and the end result is a solid ball of incredibly dense neutron matter: a neutron star. Surprisingly, the weak interactions play an indispensable role. Each proton, as it becomes a neutron, gives off two particles, a positron and a neutrino. The positrons quickly combine with the electrons in the star and disappear.
Such an event, called a supernova, is not a gentle one. A supernova can outshine an entire galaxy with a hundred billion stars!
In everyday physics and chemistry, neutrinos are of no importance at all. They can pass through light-years of lead without disturbing it one bit. Neutrinos from the sun are continually passing through the earth, through our food and drink, and through our bodies with no effect at all. But our existence is totally dependent on them. The neutrinos flying out of the supernova implosion are so numerous that, despite their feebleness, they create an enormous pressure, pushing matter in front of them. The pressure exerted by the neutrinos blows off the outer layers of the collapsing star and, in the process, sprays out the complex nuclei that were cooked before the star collapsed. So as its final act, the star in its death throes donates its complex nuclei to fill the universe with matter.

Our sun is a youngster. The universe is about fourteen billion years old, but the sun was born late in its history, only five billion years ago. By that time generations of stars had formed and died and there were already enough heavy elements to form the solar system. We are fortunate indeed that the ghostly neutrino exists—in the ordinary sense of the word.
There are multiple ways that things could go wrong with the nuclear cooking. If there were no weak interactions or if neutrinos were too heavy, protons could not turn into neutrons during the cooking. The cooking of carbon is sensitive to the details of the carbon nucleus. One of the great scientific events of the twentieth century occurred when the cosmologist Fred Hoyle was able to predict one of these nuclear details just from the fact that we are here. In the early 1950s Hoyle argued that there is a “bottleneck” in the cooking of elements in stars like the sun. There appeared to be no way for the cooking to proceed past atomic number 4—helium. Nuclear cooking usually goes forward one proton at a time to form a heavier element, but there is no stable nucleus with atomic number 5, so there is no easy way to get past helium.
There is one way out. Two helium nuclei can collide and stick together to form a nucleus with atomic number 8. That nucleus would be the isotope beryllium 8. Later, another helium nucleus could collide with the beryllium and form a nucleus with atomic number 12—good old carbon 12, the stuff of organic chemistry. But there is a fly in this ointment.
Beryllium 8 is a very unstable isotope. It decays (falls apart) so rapidly that there is not enough time for the third helium nucleus to collide before the beryllium disappears—unless an unlikely coincidence occurs. If by accident there were an excited state—a so-called resonance—of the carbon nucleus with exactly the right properties, the probability for the beryllium to capture a helium nucleus would be much higher than expected. The likelihood of such a coincidence is very small, but when Hoyle suggested that such a coincidence might solve the problem of cooking the heavy elements, experimental nuclear physicists went right to work. And BINGO, the excited state was discovered with exactly the properties that Hoyle guessed. Just a small increase or decrease in the energy of the excited carbon nucleus, and all the work of making galaxies and stars would have been in vain; but as it is, carbon atoms—and thus, life—can exist.
The properties of Hoyle’s carbon resonance are sensitive to a number of constants of nature, including the all-important fine structure constant. Just a few percent change in its value, and there would have been no carbon and no life.
6
This is what Hoyle meant when he said that “it looks as if a super-intellect has monkeyed with physics as well as with chemistry and biology.”
But again, it would do no good for the nuclear physics to be “just right” if the universe had no stars. Remember that a perfectly homogeneous universe would never give birth to these objects. Stars, galaxies, and planets are all the result of the slight lumpiness at the beginning. Early on, the density contrast was about 10
–5
in magnitude, but what if it had been a little bigger or a little smaller? If the lumpiness had been much less, let’s say, 10
–6
, in the early universe, galaxies would be small and the stars, very sparse. They would not have had sufficient gravity to hang on to the complex atoms that were spewed out by supernovae; these atoms would have been unavailable for the next generation of stars. Make the density contrast a little less than that, and no galaxies or stars would form at all.
What would happen if the lumpiness were larger than 10
–5
? A factor of one hundred larger, and the universe would be full of violent, ravenous monsters that would swallow and digest galaxies before they were even finished forming. Don’t worry; I haven’t lost my mind. The “mega-monsters” are huge black holes. Remember that gravity is the agent that works on the regions with slight excess mass density and pulls them together to form galaxies. But if the overdensities were too strong, gravity would work too quickly. The gravitational collapse of these regions would go right past the galaxy stage and evolve into black holes. All matter would quickly be gobbled up and destroyed at the infinitely violent central singularity of the black hole. Even density contrasts a factor of ten stronger could endanger life by creating too many collisions between celestial objects in the solar system.
A lumpiness of about 10
–5
is essential for life to get a start. But is it easy to arrange for this amount of density contrast? The answer is most decidedly no! The various parameters governing the inflating universe must be chosen with great care in order to get the desired result. More of Hoyle’s monkeying?
There is a lot more. The laws of particle physics include the requirement that every particle has an antiparticle. How then did the universe get to have such a large preponderance of matter over antimatter? Here is what we think happened: