A New History of Life (7 page)
Read A New History of Life Online
Authors: Peter Ward

Proteins, the last building blocks, perform four functions in Earth life: building other large molecules, repairing other molecules, transporting material about, and securing energy supplies. Proteins also modify both large and small molecules for a variety of purposes and are involved in cell signaling. There are a huge number of different proteins, and we are only now learning how these work and what they do. A new insight is that their topology, or folding pattern, is as important to their function as their chemical makeup.
All proteins used in Earth life are formed from the assembly of the same twenty amino acids. A new twenty-first-century area of research is asking an old problem: are these same twenty used because they are the best building blocks out there—or because they were common where life was first forming and then became permanently “coded” into life? In fact it looks like it is the former; they work the
best, at least according to research in 2010.
11
This group is specific to Earth, and perhaps diagnostic of Earth life.
Proteins are constructed in the cell by stringing together the various amino acids in a long, linear chain that folds into its final shape only when all its amino acids have been joined together. Sometimes they fold as they are still being synthesized. Because the assembly of amino acids into a protein is done one at a time in linear and specific order, that protein is often analogized to a written sentence, each amino acid being a word. Within its cell walls, a living cell is packed with molecules, arranged in rods, balls, and sheets, all floating in a salty gel. There are about a thousand nucleic acids and over three thousand different proteins. All of these are going about some sort of chemistry that combined makes up the process we call life. Many chemical processes can go on simultaneously in this one-room house.
There are about also about ten thousand individual spheres within the cell, known as ribosomes, which are distributed rather evenly throughout. Ribosomes are composed of three distinct types of RNA, and about fifty kinds of proteins. Also present are chromosomes, which are long chains of DNA connected to specific proteins. The DNA in bacteria is usually localized in one part of the cell, but is not separated from the other interior material by a plasma membrane, as is the case in higher forms of life known as eukaryotes, which have an interior nucleus. It can be asked just what in this cell is “alive.”
A bacterium is composed of inanimate molecules. A DNA molecule is certainly not alive, in any sense that any rational person would accept. The cell itself is composed of myriad chemical workings, each, taken alone, being but an inanimate reaction of chemistry. Perhaps nothing is alive but the whole of the cell itself. If we are to understand how life first arose, we need to find the minimum cell that can accomplish this with the fewest molecules and reactions.
One of the pressing problems in looking at this simple cell is that when examined in detail, it is in no way simple. Freeman Dyson has explicitly looked at this aspect of modern life, asking, “Why is life
(at least life today) so complicated?”
12
If homeostasis is a necessary attribute of life, and if all known bacteria contain a few thousand molecular species (coded by a few million base pairs in the DNA), it looks as if this might be the minimum-sized genome. Yet all bacteria come to us today at the end of more than 3 (and perhaps more than 4) billion years of evolution. Perhaps the simplest Earth life is among the most complicated of life forms in the cosmos.
13
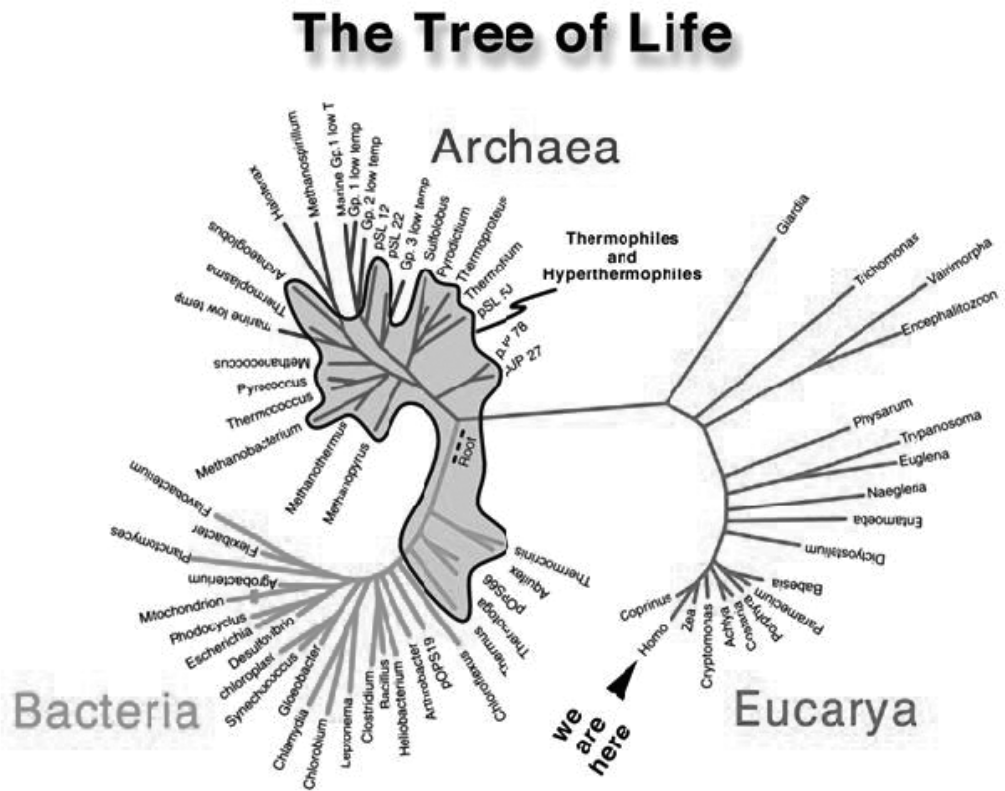
The eventual tree of life as it is now viewed. The shaded areas are those organisms that thrive in high heat. What is missing are the presumably many kinds of organisms and “pre-organisms” that evolved from inorganic chemistry step-by-step to produce the first living cell.
Forming Life: 4.2(?)–3.5 GA
On July 28, 1976, a robotic claw extended from a huge one-ton machine that only days before had completed the long, silent flight from the Earth to Mars and then had successfully landed there. The claw scooped Martian soil into the
Viking
spacecraft. This sample collection was the first time such an engineering achievement had ever been accomplished off the Earth. With this sediment now contained in its complicated interior, NASA’s
Viking
performed four basic experiments, all designed to look for chemical evidence of life or its processes. That was the entire reason for
Viking
coming to Mars: to search for life.
The initial experiments
1
raised hopes that Mars indeed harbored extant life in its soil, for it was soon found that the soil contained more oxygen than was expected, and furthermore that chemical activity of the soil at least
hinted
at a microbial presence in the Martian regolith. These first-blush experiments created such a wave of optimism in the
Viking
scientific team that one of the mission’s chief scientists, Dr. Carl Sagan, was optimistic enough to tell the
New York Times
that he thought that life on Mars, even large forms of life, was not out of the question. By large life, he meant
really
large, for in the same interview he went on to posit the existence of Martian polar bears!
But the onboard spectrograph, after carefully analyzing the Martian soil, could find no evidence of organic chemicals in the soil. Mars, as viewed from this first
Viking
lander, not only seemed dead, but inimical to life, leading to speculation that any life that might be there would soon be killed by the toxic chemicals in the soil. Sagan, ever the optimist, could now only hope that the second
Viking
lander, on that same day already orbiting Mars, would yield telltale evidence for life.
On September 3, 1976, the second lander safely parachuted onto the Martian surface at a place named Utopia Planitia. Like the first,
this huge machine functioned perfectly.
2
And also like the first, no evidence of life was found in any of the separate and crucial life-detection experiments.
Viking
had been conceived as a multi-investigative program. But while its study of the chemistry and geology of the soil and atmosphere was important, its primary mission, and most of the instrumentation crammed into the crowded spacecraft, as noted above, was dedicated to the search for extraterrestrial life.
The
Viking
results suggested that Mars was sterile,
3
and NASA began to lose interest in Mars, because NASA was and is driven by the search for life beyond Earth. NASA’s lack of interest began to benefit another branch of science, one that also was bent on studying alien worlds, and perhaps alien life: the oceanographers.
In the immediate post-
Viking
years, huge new sums went into the technology necessary for deep-ocean exploration, and soon another kind of spacecraft made its own successful descent onto an alien surface. In this case, however, life was found, but of a kind that was totally unexpected. First in the Atlantic Ocean, and then in rapid succession in the deep sea off the Galápagos Islands followed by dives in the Gulf of California, the small yellow submarine
Alvin
photographed and sampled a kind of life using a radically different source of energy than sunlight.
This discovery of deep-sea “vent” faunas would radically change our understanding of where and how life on Earth came into being, if in fact it originated on Earth at all, for there is a possibility that life formed elsewhere and then was transported to Earth. If life on Earth formed soon after our Earth coalesced into a large and ultimately habitable planet, it suggests that life is not all that hard to make. But how old really is the oldest Earth life—and where was this first life formed?
Usually when historians try to find the “first” of anything, they look into records of ever-older time units, and so it has been with the Earth historians. Their problem has been the paucity of rocks of sufficient age, and the near impossibility of a bacterium-like early cell to actually fossilize.
For more than two decades it has been axiomatic that the oldest sign of life on Earth came from a frozen corner of Greenland, at a
place named Isua.
4
No fossils were found. Instead, small minerals called apatite were reported to contain microscopic amounts of two different isotopes of carbon that showed a ratio quite similar to one that is characteristic of life today. The Isua, Greenland, rocks were well dated at about 3.7 billion years in age, and later, new dating suggested that they were even older, about 3.85 billion years, in fact, and this is the date that has long been codified into textbooks.
The date of 3.7 to 3.8 billion years old made a lot of sense to those looking for the oldest life on Earth. As we saw earlier, asteroids bombarded the Earth along with every body in the then-young solar system and other junk left over after planet formation from about 4.2 to 3.8 billion years ago. We mentioned earlier that life, while it may have formed (or have been even older that this), would have been wiped out by the process of “impact frustration.”
5
Thus the age of the Isua rocks was perfect; the heavy bombardment would have been just over, and life could start. Unfortunately for this tidy package, new instruments developed in the twenty-first century discovered that the small bits of carbon in the Isua, Greenland, samples were not formed by life at all.
6
The next-oldest life was 3.5 billion years in age, and in this case, the claim was based on fossils, not just chemical signals. Filamentous forms in an agate-like rock dated to be around 3.5 billion years in age
7
were discovered by American paleontologist William Schopf. The fossils came from a previously obscure and ancient assemblage of rocks located in one of the least habitable places on current Earth, a highly deformed rock assemblage called the Apex Chert in Western Australia. The exact geographic position of these fossils, in the dust-dry enormity of Western Australia, was the “North Pole,” a whimsical name given some years earlier because the locality is, in fact, one of the hottest places anywhere on Earth, and about as removed both geographically and especially climatically from the Arctic as a place could be.
Schopf’s discovery galvanized science, for it showed that life on this planet began very early in Earth history indeed. For almost twenty years these ancient Australian fossils were accepted as the planet’s oldest fossil life. Then these too were cast into doubt by Oxford’s
Martin Brasier, who claimed that the so-called oldest fossils on Earth were only tiny crystal traces, not life relicts at all.
8
What came next was a scientific donnybrook. Scientists on both sides unleashed attacks and counterattacks, most polite (but some less so). Back and forth it went for some years, with Schopf gradually losing ground, not only from attacks from the Oxford crew about the interpretation of the small traces in the Apex Cherts, but also soon after about the age of Apex Chert itself.
Around 2005, Roger Buick of the University of Washington claimed that even if the tiny objects in the Apex Chert are fossils at all, the rocks themselves are far younger than Bill Schopf has claimed, more than a billion years younger, in fact, which would still make them old (any fossil with billions attached to its birthday qualifies for old-age discounts), but nowhere near the oldest life on Earth. With these one-two punches, the Apex fossils were knocked out of the ring.
So matters rested until the summer of 2012, when the same Martin Brasier coauthored a paper
9
demonstrating the presence of life that is at least 3.4 million years old—which, according to the authors, makes it the oldest fossil life ever discovered. What makes the discovery even more important is the identity of the fossils themselves, all microscopic, of the size and shape of a specific kind of bacteria living on Earth today. The oldest life on Earth lived in the sea, appeared to need sulfur to live, and quickly died if exposed to even a small number of oxygen molecules. While this life is still what we might call a carbon-based life form, it brings the element sulfur front and center in our assessment of how life came about.
10
The fossils described in the Brasier paper appear to be related to minute bacteria still living on our planet—bacteria that need the element sulfur to live, and that die quickly if exposed to the thinnest whiff of oxygen. If this discovery holds, it will confirm that life on our planet began in a place utterly alien to most of the Earth today, and depended on sulfur, not oxygen.
Earth life is usually associated with the forests, seas, lakes, and skies of our present-day Earth—with the creatures living in clear air, clean blue water, on grass-covered hills. Yet the tiny fossils found
by Brasier came from an environment of temperatures far higher than those of today, with air composed of the toxic gases methane, carbon dioxide, ammonia, and not a little of the poisonous gas hydrogen sulfide.
11
It lived on a planet certainly without continents, or virtually any land of any consequence at all, beyond strings of ephemeral, volcanic isles. In this setting, life began (or arrived, a major possibility to be explored in the pages to come) and then thrived for billions of years. The majority view is that we are all descendants of this Hades on Earth cradle, bearing the scars and genes of a sulfur-rich origin of life.