A New History of Life (6 page)
Read A New History of Life Online
Authors: Peter Ward

Life reproduces.
Davies makes the point that life must make a copy not only of itself but of the mechanism that allows further copying; as Davies puts it, life must include a copy of the replication apparatus too.
Life develops.
Once a copy is made, life continues to change; this can be called development. This process is quite un-machinelike. Machines do not grow or change in shape and even in function with that growth.
Life evolves.
This is one of the most fundamental properties of life, and one that is integral to its existence. Davies describes this characteristic as the paradox of permanence and change. Genes must replicate, and if they cannot do so with great regularity, the organism will die. Yet, on the other hand, if the replication is perfect, there will be no variability, no way that evolution through natural selection can take place. Evolution is the key to adaptation, and without adaptation there can be no life.
Life is autonomous.
This one might be the toughest to define, yet is central to being alive. An organism is autonomous, has self-determination; it can live without constant input from other organisms. But how “autonomy” is derived from the many parts and workings of an organism is still a mystery.
Action and constitution are one and the same thing for the living system. The system consists of the continuous generation (and regeneration: a protein exists for only about two days) of all the processes and components that put it together as an operational unit. In this view, it is the constant reproduction and renewal of the life form that defines life itself.
This last, the temporary life-span of molecules crucial to living, and thus life, has been underappreciated as a major clue in understanding where life may first have formed. The NASA definition of life is simpler, and is from a definition favored by Carl Sagan: life is a chemical system capable of Darwinian evolution.
8
There are three key concepts to this. First, we are dealing with chemicals, and not just energy or even electronic computing systems. Second, not just chemicals, but also chemical
systems
are involved. Thus, there is an interaction among the chemicals, not just chemicals themselves. Finally, it is the chemical systems that
must undergo Darwinian evolution—
meaning that if there are more individuals present in the environment than there is energy available, some will die. Those that survive do so because they carry advantageous heritable traits that they pass on to their descendants, thus lending the offspring greater
ability to survive. The Sagan-NASA definition has the advantage of not confusing life with being alive.
What was the “driver” that caused dead chemicals to combine in such a way to be alive? Was the main driver leading to life a system of metabolism, one that only later added the ability to replicate, or the opposite of this? If it’s the first case, primitive metabolic systems—necessarily enclosed in some cell-like space—later gained the ability to replicate and incorporate some sort of information-carrying molecule. In the latter, replication molecules (such as RNA or some variant) gained the ability to use energy systems to aid in their replication, and only later became enclosed in cell. So we see a very stark contrast that this metabolism vs. replication problem poses at the chemical-molecular level: Was it proteins first, or nucleic acids first? Is either alive, and at what point does each pass from chemical reaction to chemical reactions powering life? Yet if the essential characteristic of a living cell is homeostasis—the ability to maintain a steady and more or less constant chemical balance in a changing environment—it follows that metabolism had to come first. Eating before breeding seems to be the accepted view at the present time, but as in so much dealing with the origin of life, disquieting questions remain.
The role of energy in maintaining life can now be added to our definition of life. We have already defined life as metabolizing, replicating, and evolving. But let us not consider life from an energy flow and order-disorder continuum. Just having energy is clearly not sufficient as a basis of life; there must be an interaction with the energy, and that interaction at a very basic level is needed to maintain a state of nonequilibrium order. Without energy, life goes to nonlife, so life must be something whose very definition is coupled with energy acquisition and energy dumping. Life maintains itself by having states that allow it to become progressively more orderly through the input of energy flow. Our kind of life does this by maintaining a relatively small number
of combinations of carbon, oxygen, nitrogen, and hydrogen (and some other elements in smaller volumes). Eventually, a degree of complexity and integration is reached, and maintained, that we call life. The inflow of energy must be sufficient to overcome the tendency of the chemistry within the body that we call life to revert back to its equilibrium condition—nonlife.
One of the universally accepted definitions of life is that it metabolizes. For Earth life, the primary sources of energy are from the heat of the Earth or from the sun, itself the energy arising from the sun’s thermonuclear fusion reactions. By far the most common way that life taps into solar energy is through photosynthesis. In this process, sunlight provides the energy to convert carbon dioxide and water into complex carbon compounds with many chemical bonds that store energy. By breaking these bonds, energy is released.
Life on Earth uses a variety of biochemical reactions, and they all involve the transfer of electrons. But this system works only if there is what might be called an electrochemical gradient: the steeper the gradient, the more energy that can be realized. This means that some types of metabolism yield far more energy than others, just as some kinds of environments have more energy to harvest than others. The organic (carbon-containing) compounds containing the greatest amount of stored energy are fats and lipids—long chain carbons that have much energy tied up in their chemical bonds.
Metabolism is the sum of all the chemical reactions occurring within an organism. A virus is very small; typical viruses are from 50 to 100 nm in diameter, where nm stands for nanometer, or 10
-9
meter. They come in two general types: one group is enclosed in a shell of protein, the second by both a protein shell and an additional membrane-like envelope. Within this covering is the most important part of the virus, its genome, made up of a nucleic acid component. In some there is DNA, in others only RNA. The number of genes also varies widely, with some having as few as three genes and others (such as smallpox) having more than 250 individual genes. In fact, there is a huge variety of viruses, and if they were considered alive, they would be classified across a great taxonomic spread. But common wisdom treats them as
nonliving. The viruses that contain only RNA show that RNA by itself, in the absence of DNA, is capable of storing information, and serving as a de facto DNA molecule.
9
This finding is strong evidence that there may have been an “RNA world”
10
before DNA and life as we know it originated. And there is an even more striking implication of the presence of RNA viruses.
Viruses are parasites. They are technically termed obligatory intracellular parasites, as they are unable to reproduce without a host cell. In most cases, viruses infiltrate cells of living organisms and hijack the protein-forming organelles and start making more of themselves,
turning the invaded cells into virus-producing factories. Viruses have a huge effect on the biology of their hosts.
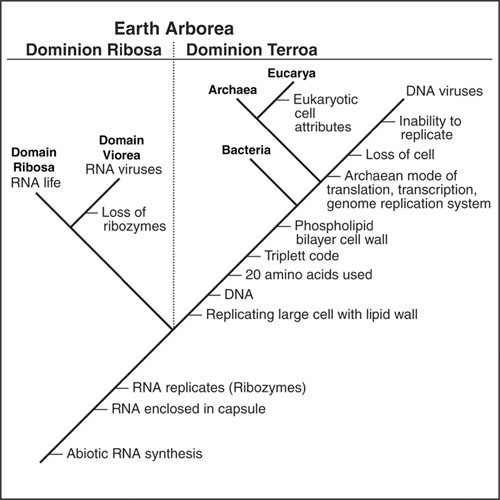
Our revised version of the tree of life, which includes viruses and now-extinct RNA life. This requires a new taxonomic category, one that is higher than domains (which are above kingdoms). RNA life is currently not definable on the accepted tree of life. (From Peter Ward,
Life As We Do Not Know It
, 2006)
The greatest argument against including viruses as alive is the fact that they are unable to replicate on their own—and thus seemingly fail this major test of whether or not an object is living. But it must be remembered that viruses are obligate parasites, and parasites tend to undergo substantial morphological and genetic changes in adapting to their hosts.
We can also ask if other parasites are alive. Parasitism, which is essentially a highly evolved form of predation, is generally the result of a long evolutionary history. Parasites are not primitive creatures. But like our viruses, they have stages that do not seem fully alive
. Cryptosporidium
and
Giardia
, both parasites on humans and other mammals, have resting stages that are as dead as any virus outside of its host. Without the hosts, these two organisms (and thousands of other species as well) will not live, perhaps cannot be classified as living. Yet when in their hosts, they show all the hallmarks of life as we know it: they metabolize, they reproduce, and they undergo Darwinian selection. But if we accept that viruses are alive, and this is increasingly accepted, we must radically reassess the tree of life as it is currently accepted.
In studying life on Earth, two questions can be posed: What is the simplest assemblage of atoms that is alive? And what is the simplest life form on Earth, and what does it need to stay alive? To answer these questions we must look at what current Earth life needs to attain and maintain the state of life described above. To do this we must briefly digress into chemistry of the materials that all Earth life uses to attain and then maintain life.
Of all the molecules making up Earth life, perhaps none is more important than water, and water in a single phase: it has to be liquid water, and not ice or water vapor (a gas). Earth life is composed of molecules bathed in liquid, and while the number of molecules that
can be found in life is staggeringly large, in fact there are only four main kinds of molecules used by Earth life: lipids, carbohydrates, nucleic acids, and proteins. All of these are either bathed in liquid, in this case water with salt in it, or serve as an outer wall to contain the other molecules and water.
Lipids—what we call fat—are key ingredients in the cell membranes of Earth life. They are water resistant due to an abundance of hydrogen atoms, but they contain few oxygen and nitrogen atoms. Lipids are the major components of the cell boundary or wall that separates the outside environment from the fluid-filled interior of what we call life. These membranes, although delicate, provide control of substances in and out of cell.
Carbohydrates are the second major class of structures that Earth life is made of, and they are what we informally call sugars. By linking together a number of them, we can form a polysaccharide, which means “many sugars.” Sugars, be they linked or single, are important building blocks in that they can be combined with themselves or with other organic and inorganic molecules to form larger molecules.
Sugars are also important in forming the next category of building block, nucleic acids. This group contains the stored genetic information of any cell. They are giant molecules that combine sugars to nitrogen-containing compounds called nucleotides, themselves formed from subunits called bases, phosphorus, and more sugars. In this arrangement the bases are crucial, for they become the “letters” in the genetic code.
DNA and RNA are sugars that are among the most important of all molecules of life. DNA, composed of two backbones (the famous double helix described by its discoverers, James Watson and Francis Crick), is the information storage system of life itself. These two spirals are bound together by a series of projections, like steps on ladder, made up of the distinctive DNA bases, or base pairs: adenine, cytosine, guanine, and thymine. The term “base pair” comes from the fact that the bases always join up: cytosine always pairs with guanine, and thymine always joins with adenine. The order of base pairs supplies
the language of life: these are the genes that code for all information about a particular life form.
If DNA is the information carrier, a single-stranded variant called RNA is its slave, a molecule that translates information into action—or in life’s case, into the actual production of proteins. RNA molecules are similar to DNA in having a helix and bases. But they differ in usually (but not always) having but a single strand, or helix, rather than the double helix of DNA.
Why the enormous complexity of DNA and RNA? The answer lies in the need for information to first build (blueprints) and then maintain the many tasks that staying alive requires. DNA is the blueprint, instruction manual, repair manual, and directions for building copies of itself and all that it codes for. In computer terms, DNA is the software, in that it carries information but cannot itself act on the information. Proteins can be thought of as the computer’s hardware, needing the DNA software to provide information of when and where specific chemical changes should occur in time and space, and to produce material necessary for life. RNA has the interesting characteristic of being either hardware or software, and in some cases both at the same time.