Why Is Milk White? (22 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho

The stomach makes the acid by separating neutral salts into an acid and a base. The acid is hydrochloric acid, and the base is bicarbonate. The bicarbonate stays in the stomach lining, to protect the cells there from the acid. The acid can then digest the food without digesting the stomach itself.
Sometimes the acid in your stomach gets into your esophagus, the tube leading into the stomach. If you have eaten too much and lie down, this is more likely to happen. The esophagus does not have the protective bicarbonate that the stomach lining has, so the acid hurts the delicate esophageal walls. This is called heartburn, or acid reflux. You have to sit up, or stand up, until your stomach empties enough to lie down and not spill acid into the esophagus.
Another way an acid can be “not good” is if it is no longer as acidic as it needs to be. The acid in batteries is needed for them to work properly. If it is diluted or neutralized, then the batteries won't work.
Matter is anything that has mass and takes up space. Mass is what gives things weight on Earth. If something feels heavy, it is matter. But the helium in a balloon is also matter. It only floats because the air around the balloon is heavier.
Most of the matter you encounter every day is made up of atoms. Your body, the ground under your feet, and the air you breathe are all made of atoms.
Inside the atoms, there are protons and neutrons in the nucleus and electrons in clouds around the nucleus. All of these particles have mass and take up space.
Protons and neutrons are in turn made up of even smaller particles, called quarks. And there is a whole zoo of particles that are
seen when atoms smash together, with names like muons, taus, and neutrinos.
There are also things that aren't matter. These are called particles, but they have no mass and they take up no space. They carry force, and matter reacts to these forces. The photon carries the electromagnetic force and allows us to see, to feel heat, to send radio signals, and to take X-ray pictures. Other force-carrying particles are bosons and gluons.
Scientists also suspect that there is a lot of matter in the universe that we cannot see. We call it
dark matter,
and it is responsible for keeping galaxies from flying apart. It may be made up of particles that we have not yet detected. Some of it is undoubtedly neutrinos and black holes, but there seems to be far too much of it to be made up of just those things we know about.
Nothing. Chemistry is the study of how atoms combine with other atoms. Gluons are force-carrying particles that hold quarks together to form protons and neutrons in atomic nuclei.
While we would not have atoms without gluons, gluons do not participate in the reactions that join atoms together to form molecules. What holds atoms together is the electromagnetic force, in the form of positive charges in the nucleus and negatively charged electrons.
Electrons move easily from one atom to another. Protons (hydrogen nuclei) are easily exchanged in reactions between acids and bases. But gluons stay inside the nucleus and don't play any part in sticking one atom to another.
There are two hydrogen sulfates.
There is the hydrogen sulfate ion (also called the bisulfate ion), which has a sulfur atom, four oxygen atoms, and a hydrogen atom.

It has an extra electron (which makes it an ion) that hangs around one of the oxygen atoms.
If that lone extra electron attracts a proton (making a hydrogen atom) you get the other hydrogen sulfate. Because it has two hydrogens, it would be dihydrogen sulfate.
A more common name for this molecule is sulfuric acid. This is a powerful acid that is widely used in industry. It is the acid used in lead-acid car batteries.
A similar sounding molecule is hydrogen sulfide.
Hydrogen sulfide is a poisonous gas and is familiar as the smell of rotten eggs.
While sulfuric acid is a very strong acid, there are some acids that are even more powerful. In fact, if an acid is more acidic than sulfuric acid, it is called a
superacid.
Most of the acids you might encounter are molecules that react with water by breaking up and donating a proton to the water. A proton is a hydrogen nucleus, and the water molecule that accepts it becomes a hydronium ion, H
3
O
+
.
Hydrogen chloride (HCl), for example, breaks apart in water to form a negative chloride ion Cl
-
and donates its hydrogen nucleus to water to form the hydronium ion H
3
O
+
.
A strong acid is an acid that loses all of its hydrogen nuclei (protons) in water. Weak acids, like the acetic acid in vinegar, don't lose all of their protons in water, so some of the molecules stay intact.
The carborane superacid H(CHB
11
Cl
11
) is a million times stronger than sulfuric acid. It donates a proton very easily.
But while the ease with which an acid donates a proton is how the strength of an acid is defined, that is not what makes the acid corrosive, which may have been the main point of the question. What makes an acid corrosive is what is left behind after the proton leaves.
In the case of hydrofluoric acid, HF, what is left is the very reactive fluoride ion, and hydrofluoric acid reacts with almost anything. It even reacts with glass, so it can't be stored in glass bottles.
In the case of carborane, what is left behind is a very stable ion that does not react with much. So although it is the strongest of the superacids, it can be stored in a glass jar.
But the strong superacid fluoroantimonic acid (HsbF
6
) is both an extremely strong superacid and quite corrosive.
Another famously corrosive acid is a mixture of one part nitric acid to three parts hydrochloric acid. It is called
aqua regia
(“royal water”), because it can dissolve the noble metals gold and platinum.
Hydrogen chloride (HCl) is a gas. It is made up of two atoms, hydrogen and chlorine. It dissolves very easily in water, where it breaks down into a positively charged hydrogen nucleus (a proton) and a negatively charged chlorine ion. The proton immediately reacts with a water molecule to form the hydronium ion H
3
O
+
.
The result of dissolving the gas in water is called hydrochloric acid. It is a very useful acidâit cleans tarnish and rust off metals, it etches concrete, and it is used in swimming pools to neutralize the water (which is often slightly basic due to other chemicals in the water).
But hydrogen chloride gas is also interesting by itself. It will react with ammonia vapors in the air to form ammonium chloride, which is a solid. A fun thing to do
(with adult supervision)
is to put a little diluted hydrochloric acid on one paper towel and some ammonium hydroxide solution on another paper towel and bring them together. The vapors combine in the air, and the solid ammonium chloride particles look like smoke. (See the “Smoking Hands” project on
page 4
for another version of this trick, and some
important safety guidelines
.)
The chlorine atom in hydrogen chloride holds on to electrons very tightly. The electron that would normally be associated with the hydrogen is strongly attracted to the chlorine and ends up spending most of its time on that side of the molecule.
This behavior leaves one side of the molecule positive and the other side negative, forming what is called a polar molecule. Water is also a polar molecule, and polar molecules attract one another because of their electrical charges. Hydrogen chloride gas combines with water vapor in the air to form hydrochloric acid vapor.
It's a matter of balance. In chemistry, the word for balance is
equilibrium.
If something is in equilibrium, the amounts of things in it stay unchanged.
A snowflake is in equilibrium between solid water and liquid water. The bulk of the snowflake is solid, but at the surface there is a layer of liquid water, and some of that surface water escapes to become the gas water vapor. Molecules of water can also escape directly from the solid ice into the air to become water vapor without becoming a liquid first.
What keeps the snowflake intact is equilibrium. For each water molecule that leaves the ice to become water or water vapor, there is a molecule of water or water vapor that crystallizes onto the surface of the ice.
If the balance is disturbed, more atoms will solidify, forming more ice, or more atoms will liquefy, forming more water. The balance (or shift in the equilibrium) can be changed by changing the temperature or changing the pressure.
Heat is the motion of molecules. If heat is added to something, it raises the temperature, which is the speed of the molecules. In ice, the molecules are bouncing around against one another, vibrating back and forth. If the speed at which they bounce is increased, they can jostle loose and become liquid water.
If the pressure is increased, the water molecules on the outside of the ice are pushed back toward the ice, where they have a better chance of sticking to the other molecules there. So increasing the pressure makes more ice, and decreasing the pressure makes more water or water vapor.
Salt melts the ice. When you read about snow melting in the answer above, you saw that there is a balance between how many molecules froze onto the ice and how many molecules in the ice melted into the surrounding water. You saw that you could add heat or lower the pressure to tip the balance in favor of melting.
There is another way to tip the balance. Salt water doesn't freeze until it reaches almost -6° Fahrenheit (-21° Celsius). If salt is added to the water at the surface of the ice, the ice still melts at
the same rate (that hasn't changed), but the water no longer freezes onto the ice until the temperature drops to -6° F.
It takes heat to melt ice. The ice can get that heat from the air or water around it, if that is warmer than the ice. The ice stays the same temperature (the freezing point), but the salt water around it gets colder as it loses heat melting the ice. Eventually the salt water reaches -6° F, and we are back in equilibrium again, with water freezing onto the ice as fast as it melts off it.
So if the temperature outside is below -6° F, salt won't melt the ice. You have to use something that freezes at an even lower temperature. A calcium chloride solution freezes at -20° F (-29° C), so it is used instead of the cheaper salt when the temperature is really low.
and â¦
Does ice weigh more than water?
No. I'm assuming the question refers to equal volumes of each, so it is really asking which is denser, water or ice.
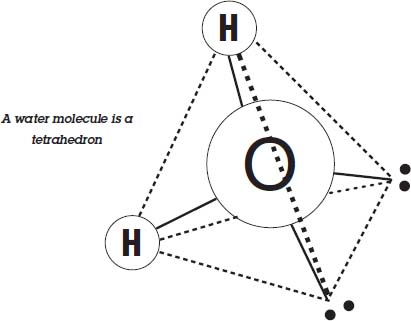
You can see that ice floats in water, so it must be less dense than the water. To understand why that might be, you need to look closely at a molecule of water.
A water molecule forms a lopsided tetrahedron. The two hydrogens are at two corners of the pyramid, and the other two corners have pair of electrons. The electrons repel one another, and that causes the tetrahedron to be somewhat lopsided, so the angle between the two hydrogens is 104.5° instead of the 109.5° that a perfect tetrahedron would have.
The hydrogens are slightly positive, and the electron pairs are slightly negative, so if it was cold enough the molecules would stick together with a hydrogen of one molecule attracting the electron pair of another. This arrangement makes a six-sided crystal, and the molecules are about 9 percent farther apart than they would be if they were just randomly jumbled together, as they are in liquid water.