Why Is Milk White? (16 page)
Read Why Is Milk White? Online
Authors: Alexa Coelho


People wonder about the unusual things in life, but they wonder even more about the annoying things. Though they might never ask why a flower smells so nice, they almost always wonder what is making that awful smell.
Humans' main sense for detecting molecules is in their noses. Their tongues have only a few chemical sensors in them, and their other senses have even fewer. It is difficult to tell strong tea from vanilla extract by sight, touch, or sound. But the
nose
knows.
and â¦
Why do chemicals smell?
There are two mechanisms that cause odors that smell or stink.
Some things strongly irritate mucus membranes in your nose, eyes, and throat. Caustic chemicals such as ammonia, chlorine, and acid vapors stimulate pain sensors and make you jerk away from the odor.
But most smells are more subtle and are detected by special cells in your nose. An odor molecule may stimulate more than
one cell, and different odors can be recognized by combinations of receptors. Each olfactory cell in the nose has a nerve that sends the information to the brain. Humans have about 40 million of these cells. Dogs have 50 times as manyâabout 2 billion. Humans have about 350 genes for odor receptors and can distinguish about 10,000 different odors.
If you are exposed to a particular smell for a while, you become used to it, and your body no longer detects it. The brain seems to be interested in new smells more than common smells, such as your own body odor. Women tend to have more sensitivity to odors than men do. Pregnancy enhances this effect. Humans' ability to detect odors decreases as they age.
Your ability to smell things helps you tell which foods are good to eat and which foods are rotten. It keeps you away from dangerous places that have high levels of bacteria or harmful vapors. Most of the things that smell bad are harmful to you or indicate that something may be harmful.
Skunks use their odor to defend themselves and keep predators away. Skunks spray a mixture of chemicals from special glands under the tail. These chemicals mostly contain molecules with a thiol group (a sulfur attached to a hydrogen).
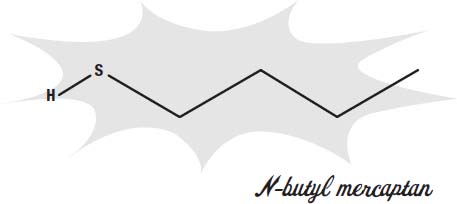
These chemicals are known as
mercaptans.
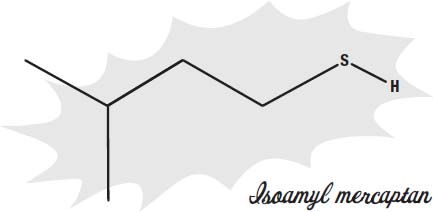
These are molecules that humans can sense when even one molecule is present among 10 billion air molecules.
A modification of these molecules is also present, where the thiol group has reacted with acetic acid to form what is called a
thioacetate.
These molecules have no smell but break down into the mercaptans when they get wet. This is why a dog that has been sprayed by a skunk smells worse when the air is humid.
Knowing that altering the thiol group removes the smell allows us to find remedies for skunk spray. If you oxidize the molecules with bleach, they lose their aroma. This is fine for washing clothes, but to wash a pet, you need something milder than chlorine bleach.
Hydrogen peroxide will work, but only if it is in an alkaline solution. So if you wash the dog in a mixture of baking soda and peroxide, much of the odor goes away. Repeating the wash helps, as does a final wash with a scented shampoo.
Your feet sweat, just like the rest of you does. Sweat itself is normally odorless, but it contains nutrients for bacteria and provides a nice warm, moist place for them to live.
Sweat contains amino acids and proteins (which are made of amino acids). Bacteria can break these down into simpler molecules as they eat them. One of the simpler molecules is
propionic acid.
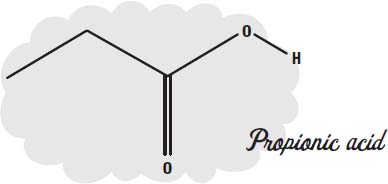
Propionic acid has the same structure as the acetic acid in vinegar, except it has an extra carbon atom added on the left side. It has a strong vinegary odor.
Another similar breakdown product of amino acids by bacteria is
isovaleric acid.
It also has a strong smell, and it is one of the molecules bacteria produce when they break down amino acids during the manufacture of cheese. This molecule is one of the main contributors to the aroma of cheese.
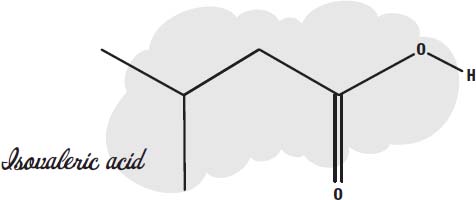
So if your socks smell faintly of vinegar or cheese, you can blame the bacteria we use to make vinegar and cheese. They are at work in your shoes.
When is your tongue like your socks? When it harbors bacteria that make bad smells.
Almost all of the odor in bad breath (called
halitosis
by doctors) comes from your tongue. Some may come from the gums if you have gum disease. Some comes from the rest of the mouth or the throat, nose, or stomach, but by far the most fertile ground for breeding halitosis bacteria is the broad top surface of the tongue.
The top of your tongue has many little crevices that bacteria can live in. Most of the bacteria that cause bad breath are
anaerobes,
bacteria that grow best without air. As you sleep, they grow undisturbed, and you wake up with morning breath.
As the bacteria digest food particles and dead skin cells on the tongue, they create waste products that include the hydrogen sulfide that gives rotten eggs their odor, the methyl mercaptan that is present in skunk scent, and other sulfur-containing small, volatile molecules that result from the breakdown of sulfur-containing proteins.
Amino acids that don't contain sulfur also break down into smelly molecules. Tryptophan breaks down into
indole
. It is one of the molecules that gives fecal matterâanimal droppingsâits odor.
Another product of tryptophan breakdown is
skatole,
which is just indole with an extra methyl group added. It is also found in feces and contributes to that characteristic odor.
So please do us all a favor: brush the top of your tongue in the morning when you brush your teeth.

Nail polish contains several ingredients that give it its strong odor. Some of those ingredients are the solvents used to keep the plastic liquid until it dries on your toenails. Butyl acetate, ethyl acetate, and toluene are examples. Butyl acetate gives bananas their smell and is also used to flavor candies.
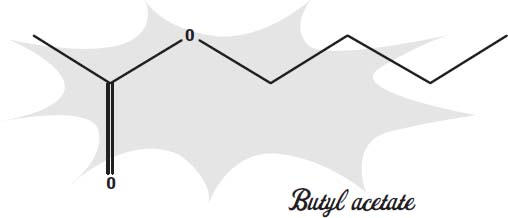
Ethyl acetate is similar ester that smells like pears. When these two molecules are present in huge amounts, they can have a very strong odor.
Toluene is derived from petroleum and gives paint thinner its characteristic smell. It is also used as the fluid that fuels some cigarette lighters.
Nail polish may also include camphor, a strong-smelling molecule that is used to keep the plastic flexible. Camphor is now made synthetically, but it was originally found in the camphor laurel tree that grows in China and Borneo. It is sometimes used as a moth repellant (the tree probably produced it as an insecticide). It gives Vicks VapoRub its strong scent.
Nail polish remover also has an interesting smell. It is mostly acetone, a strong organic solvent that is good at dissolving many plastics, glues, and paints.
There are several types of glues. Glues that use a plastic or rubber dissolved in a petroleum-based solvent will smell like the solvent. For example, rubber cement is latex rubber dissolved in n-heptane.