Simply Complexity (6 page)
Authors: Neil Johnson

This law tells us that no matter how hard we might try to stop it from happening, the Universe as a whole is heading toward total disorder. In other words, all the objects in the Universe – which ultimately are just collections of molecules – are heading toward total disorder. In short, the future is just one big messy soup of molecules. Now, I am sure that someone is thinking “I am made of molecules. So does it also include me and my molecules as well?” Unfortunately, yes it does. Indeed the phrase “ashes to ashes and dust to dust” captures the whole degenerative process very well. We will all eventually die and our bodies will then gradually decay, decomposing into various pieces and ultimately constituent molecules. Our molecules will then eventually find themselves spread out over the entire surface of the Earth, and then ultimately throughout the entire Universe as the Earth itself disintegrates and the Universe continues its unstoppable march toward increased disorder.
Wait a minute though – we can order files, and we can order the state of a ruler. So doesn’t the fact that we can create such pockets
of order mean that order has actually increased, and hence that this fundamental law of physics is wrong, and hence we are saved? Unfortunately not. We are certainly able to create temporary pockets of order in certain places and at certain times, if we feed in the right amounts of energy and effort from the outside. However it turns out that this local increase in order comes at the expense of a decrease in the amount of order in your body and in your immediate environment. As you reorder the files or make the ruler stand upright, for example, you are using energy – and some of this energy is lost as heat since you are effectively doing some exercise. And adding heat to your environment means that you are increasing the disorder in the air molecules around your body. In fact it is even worse than this – the disorder which you create as a by-product of your reordering of files or balancing of rulers will always be greater than the amount of order which you manage to create. In other words, the law is correct in that the overall disorder in the Universe increases. So although we humans can invent stories, build buildings, and can even create new lives by giving birth, each of these acts will actually destroy more order in the rest of the Universe than it can possibly create in the resulting book, building or baby.
Depressing? Actually it was a physicist called Ludwig Boltzmann who came up with the pioneering insights into this effect of increasing disorder – and he ended up committing suicide in 1906 by hanging himself while on vacation.
But we shouldn’t be too glum. It turns out that some disorder is good for us. In fact it is doing us all heaps of good right at this very moment, at various levels of human biology. In particular, it is helping us all breathe easier.
Imagine yourself back in your office, happily getting your breath back after the filing fiasco. You are breathing in air molecules, without a second thought that there may be a sudden shortage of them in the vicinity of your nose. But should we really be taking our next breath of air for granted? It turns out that disorder is our
savior – and we can understand this in terms of our analogy with piles of files, where the files now represent air molecules. In our filing scenario, we had originally arranged a set of files A, B, C, etc. in a pile on a single shelf. Now let’s generalize things slightly, and imagine instead that there are three possible shelves where the files could be placed, with each shelf being the in-tray for a particular employee. Our three employees are Ms. X, Mr. Y and Mrs. Z. If there was only one file, say file A, there would be three possible shelves upon which it could be placed. In other words, there would be three possible arrangements of one file among the three shelves. Specifically, file A could be on shelf X, on shelf Y, or on shelf Z. But imagine that there are three files. Since air molecules are all the same, and it is air that we are trying to understand, we will consider the case where the three files are all the same. So let’s think about putting them somewhere on the three shelves.
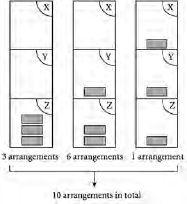
Figure 2.2
Number of possible arrangements of three identical files in a filing cabinet containing three shelves X, Y and Z
Figure 2.2
shows that there are ten ways of arranging the three identical files among three shelves. Looking at the left-hand diagram in
figure 2.2
where all three files are on the same shelf, there are three possible places for this pile of three files: all three files on shelf X, all three on shelf Y, or all three on shelf Z. Hence there are three arrangements. Things are a little bit more tricky in the
middle diagram, since the pile of two files could go in any of three shelves while the remaining file will have the possibility of either of the other two shelves. Hence there are 3 × 2 = 6 possibilities. The right-hand diagram is easier: there is only one way of arranging the three files so that each shelf has just one file.
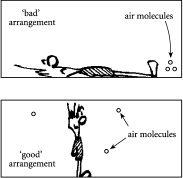
Figure 2.3
Air, air everywhere? Top shows an arrangement where the air molecules are all piled in one region of the room, like having a set of files being stuck on one particular shelf. This “bad” arrangement is very rare. Bottom shows an arrangement of air molecules where they are spread throughout the room, like having a set of files being spread out over many different shelves. This “good” arrangement is very common.
Suppose that you are lucky enough to be off on vacation again, and that your boss has purposely distributed the three identical files A, B and C, in a particular arrangement among the various employee shelves X, Y and Z. Each day, your careless summer intern comes into your office, takes out a file, and then replaces it at random among the three shelves. Before long, your boss’s original arrangement is lost and disorder once again rules. Let’s focus on the left-hand diagram of
figure 2.2
. This tells us that there are certain arrangements which correspond to all the files being in the same place at the same time. If we imagine that the files are air molecules, and that the filing cabinet represents the office itself, this particular arrangement shown in the left-hand side of
figure 2.2
would correspond to all the molecules being piled up in
one region of the office. So if you happened to be in another part of the office, as in the top of
figure 2.3
, there would be no molecules within sniffing distance of your nose – and that of course would spell trouble for air-breathers such as ourselves.
So, given that such bad (i.e. unhealthy) arrangements can arise, why aren’t we all dropping like flies? The clue to the answer lies within
figure 2.2
, which shows that there are relatively few arrangements where the objects will all be piled up in one region. Hence the chances of an arrangement arising in which all the air molecules in a room are piled up far away from you is remote. It could happen, but it certainly won’t happen very often given the fact that there will always be many billions of air molecules in a room. In fact, I have never heard of anybody experiencing such an effect. In short, disorder saves the day – it guarantees our next breath regardless of where we happen to stand.
But what would happen if an evil scientist did manage to momentarily arrange all the air molecules into one part of the room – would we then die? No, we would hardly even notice it. Air molecules have energy and hence move around and bounce off each other. Therefore it wouldn’t be long before all the possible arrangements had been explored – just as with the careless intern and the files. In physics-jargon, we say that the system explores its
state-space
very quickly. So thanks to the very rapid way in which air molecules disorder themselves and the fact that molecules have no intrinsic feedback effect and therefore cannot easily reorder themselves, we never have to worry where our next breath of air is coming from.
I do however feel duty-bound to add a slightly scary caveat. If, for some bizarre reason, air molecules were ever to gain the ability to process information like drivers and traders, and hence introduce feedback into the system, we might see spontaneous order appear in a roomful of air just as it does for traffic jams and market crashes. And then we really would have to worry.
Complex Systems tend to be “open”. In other words, the system interacts with what is around it. But it turns out that the
way
in
which it interacts with its surroundings can actually bias the frequency with which particular arrangements of its constituent objects are observed. We humans again provide a good example of this. Although the molecules in our body could in principle start flying around all over the place, they stay within the confines of our body as a result of the interactions that they experience with each other and with the outside world. By eating, drinking and sleeping, we each maintain our body in an alive state – hence we keep our bodies from decaying and thereby keep our body’s molecules on the “order” side of the order/disorder divide.
This tells us that the external conditions that a Complex System such as our body experiences can play a major role in biasing the arrangements of objects that are then seen. In the case of our own bodies, this means that the only arrangements of molecules that we observe are the ones in which our own molecules lie within the confines of our own body. A similar situation could arise with our filing problem from
figure 2.2
: in other words, we could in principle bias the arrangements such that the three files always remain in the same place if we were to put in enough effort and energy.
The fact that biases in the arrangements of objects can arise as a result of external conditions is very important for our understanding of which emergent phenomena are likely to arise in a given Complex System. This is because such biases directly affect which arrangements arise more frequently, and hence are more likely to be observed. Likewise, such biases can also prevent some arrangements from ever occurring. By developing an understanding of the biases introduced by external conditions, we should therefore be able to improve our chances of accurately predicting the system’s future behavior. For example, the decision to close off a particular road can dramatically change the frequencies and locations at which traffic jams appear in a particular road network. So given its potential importance for understanding the future evolution of a Complex System, let us explore this biasing effect further using our filing problem:
Three files, three shelves, and one careless intern:
When we discussed
figure 2.2
, we assumed that employees X, Y and Z were the same in that they were all equally likely to receive a given file. This would be the case, for example, if they all had the
same job and worked for the same number of hours. But now suppose that their work contracts are such that X works less hours than Y, who in turn works less hours than Z. Hence X will get less work and hence less files than Y, who will in turn get less files than Z. As a result, certain arrangements of files should arise more often than others. Going back to
figure 2.2
, we can see that the particular arrangement shown in the middle is probably the most likely. By contrast, if we had said that X and Y were each employed for only three hours per week, but Z was full-time, then the particular arrangement shown on the left would be more likely.
In the above examples, the external condition imposed by the three employees’ contracts drives the system toward certain arrangements and away from others. In other words, we have shown how biases in arrangements of objects can arise as a result of external conditions. There are of course many other ways in which the external conditions can bias the likely arrangements. Imagine that there is a government or trade union rule which says that no employee can handle more than one file per week. The only arrangement that should then be seen is the one on the right-hand side in
figure 2.2
. Another way in which arrangements can be biased arises when the external constraint corresponds to a rule which restricts how some particular subset of the objects can be arranged. In physics-jargon, this effect is called frustration. Now if you are one of the unlucky ones who has to put up with complicated office dynamics, this effect sounds pretty close to home. But it also turns out to be quite a general emergent phenomena in real-world Complex Systems – in particular, the ones which involve collections of objects competing for some kind of limited resource.