Heat
Authors: Bill Streever



In accordance with the U.S. Copyright Act of 1976, the scanning, uploading, and electronic sharing of any part of this book without the permission of the publisher constitute unlawful piracy and theft of the author’s intellectual property. If you would like to use material from the book (other than for review purposes), prior written permission must be obtained by contacting the publisher at [email protected]. Thank you for your support of the author’s rights.
For my companion and wife, Lisanne Aerts,
who is always eager to go somewhere new
and unusual no matter what the temperature
may be. And for my son, Ishmael Streever, who
will eat the hottest of peppers without hesitation
in exchange for a nominal fee.
Heat can also be produced by the impact of imperfectly elastic bodies as well as by friction. This is the case, for instance, when we produce fire by striking flint against steel, or when an iron bar is worked for some time by powerful blows of the hammer.
—Hermann von Helmholtz,
On the Conservation of Force
(1862)
Eating coals of fire has always been one of the sensational feats of the Fire Kings, as it is quite generally known that charcoal burns with an extremely intense heat.
—Harry Houdini,
The Miracle Mongers: An Exposé
(1920)
Throughout
Heat
, I use degrees Fahrenheit because it is the temperature scale most familiar to most readers. To convert from degrees Fahrenheit to degrees Celsius (or centigrade), subtract 32 and then multiply by 5/9. Here are a few examples, crossing the range of temperatures explored in
Heat
:
| Degrees Fahrenheit | Degrees Celsius |
| 0 | –18 |
| 32 | 0 |
| 80 | 27 |
| 125 | 52 |
| 212 | 100 |
| 2,200 | 1,204 |
| 11,000 | 6,093 |
| 7 trillion | Just under 4 trillion |
O
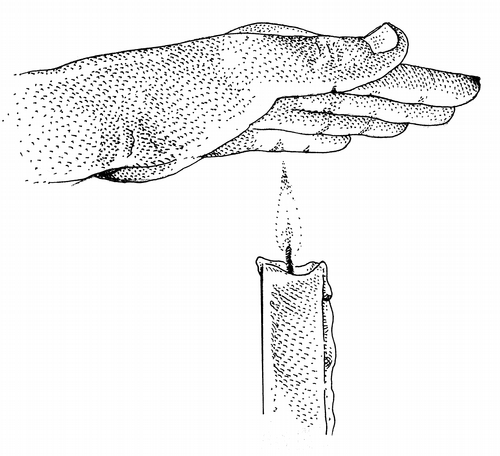
n a table in my suburban Anchorage home: a stubby red candle, matches, an electronic thermometer, a bowl of snow, and my ungloved left hand.
I look at the palm of my left hand: for now unburned, soft and uncalloused, scarred from a long-ago accident with a knife. On the back of my hand: fine blond hairs and early wrinkles of aging skin.
I strike a match, hold it while the flame stabilizes, and touch it to the candle's wick.
I move the back of my hand through the flame and smell melting hair. Hair is keratin, the same stuff in feathers and hooves and fingernails and baleen, a complicated protein, its long molecules coiled and folded upon themselves. The smell is that of protein molecules uncoiling and unfolding, coming unglued, losing their three-dimensional structure, denaturing.
Firefighters sometimes talk of skin melting. A Chicago firefighter struggled to carry a woman from a burning house. “She was slipping out of my hands because my skin was melting,” he later told an interviewer. “On my left hand, the melted skin had fused together my fingers.”
I pass my palm quickly through the flame. I feel a mild sensation of heat. One of the lessons of heat: duration of contact matters.
From Herman Melville's Mr. Stubb, lover of rare meat and second mate aboard the
Pequod
during her pursuit of Moby Dick, on how to cook a whale steak: “Hold the steak in one hand, and show a live coal to it with the other.”
Two centuries before Melville, scientists tried to understand heat. The German scientists Johann Joachim Becher and Georg Ernst Stahl promoted the phlogiston theory. Flammable materials contained phlogiston, a colorless substance with neither taste nor odor. Burning removed phlogiston. A burning candle became dephlogisticated. A steak held to a live coal long enough could become dephlogisticated. My hand, held in the flame long enough, could become dephlogisticated.
A century later, the great French scientist Antoine Lavoisier, the man who named oxygen and hydrogen, dephlogisticated the phlogiston theory. He argued that heat was a subtle fluid. He called the fluid “caloric.”
Antoine Lavoisier was as wrong as Becher and Stahl.
Slowly, I move my hand, palm down, toward the flame. At a distance of six inches above the flame, the heat becomes uncomfortable. It becomes a threat. I pull back.
During Melville's lifetime, Michael Faraday played with candles. Faraday is best known for his experiments and discoveries in magnetism and electricity, but he cherished the burning candle as a lecture topic. In 1860 he published his lectures as
The Chemical History of a Candle
.
“There is no more open door by which you can enter into the study of natural philosophy,” Faraday wrote, “than by considering the physical phenomena of a candle.”
With my electronic thermometer, I measure my candle's flame. It is an infrared thermometer, a pyrometer, capable of measuring heat from a distance, capable of seeing heat as the eye sees light. It looks beyond the visible spectrum, seeing wavelengths longer than the reddest of reds. The thermometer has the shape of a toy pistol, a gray phaser with a yellow trigger. Heat waves enter through the barrel, pass through a lens, and are focused on a thermopile. The thermopile converts heat waves to electricity. Circuitry converts the electricity into a digital readout. The digital readout says 1,350 degrees.
Faraday, in his candle lectures, described the candle flame as a hollow shell. Inside the shell there is no oxygen and therefore no fire.
I attach a steel-tipped probe to my thermometer, bypassing its optics. I push the probe into the unburning core for a reading of 1,200 degrees. At the tip of the flame, where oxygen meets hot gas, where ignition creates a yellow flare, I read 1,360 degrees, only 10 degrees off from the infrared reading. This is where I plan to bathe my hand, a five-second intentional exposure.
Twenty-three years after the publication of
Moby Dick
and seven years after Faraday died, Harry Houdini was born. Houdini grew up to become a Vaudeville star, an escape artist and illusionist, a film director, a skeptic, an exposer of lesser magicians, and a theatrical pyromaniac. Among his many offerings: instructions for cooking a steak. Houdini's instructions involved something more than a hot coal. To cook a steak, he required a cage. The cage should be made of iron, with dimensions of four feet by six feet. Its bars should be wrapped in oil-soaked cotton.
“Now take a raw beefsteak in your hand and enter the cage,” Houdini wrote.
A stagehand ignites the oil-soaked cotton wrapped around the bars. Smoke and flames hide the performer from the audience. Inside, the performer hangs the steak from a hook above his head and then lies facedown on the bottom of the cage. The performer's cloak, including a hood, is made from asbestos fabric. The performer breathes through a hole in the bottom of the cage. The heat rises and cooks the steak.
“Remain in the cage until the fire has burned out,” Houdini instructed, “then issue from the cage with the steak burned to a crisp.”
I stare at the candle's brilliant yellow, its flickering blue, the molten wax at its base. From a society that promotes meditation: “Relax your body and mind as much as you can, and watch the candle flame. Do not strain your eyes while focusing on the candle flame, as the focusing you will be doing is in your mind, not in your eyes. Clear all of your thoughts, and focus them only on the flame.”
Looking into the flame, I cannot clear my thoughts. I think of Faraday. He mentioned the “sperm candle, which comes from the purified oil of the spermaceti whale.” During lectures, he ignited alcohol, phosphorous, zinc, hydrogen, a reed soaked in camphene, and the spores from club moss. He burned everything in reach.
John Tyndall was a friend and admirer of Faraday, a user of a primitive thermopile, and Faraday's successor as director of the Royal Institution. Tyndall was intimately familiar with Faraday's candle lectures. He discovered that carbon dioxide can trap heat in the atmosphere. And he knew that heat was not a substance. It was not a fluid, subtle or otherwise. It was an expression of molecular motion.
From Tyndall, in 1863: “The dynamical theory, or, as it is sometimes called, the mechanical theory of heat, discards the idea of materiality as applied to heat. The supporters of this theory do not believe heat to be matter, but an accident or condition of matter; namely, a motion of its ultimate particles.”
Tyndall was a serious man, a man of science, and we have no record of him bathing his hand in a candle's flame. The title of his 1863 book:
Heat: A Mode of Motion
.
Quickly this time, I move my palm down into the candle's flame. I feel intense pain. I hold a world of pain in the palm of my hand.
Doctors sometimes use numerical pain scales, instructing patients to self-assess. Zero is no pain, five is moderate pain, and ten is the worst imaginable pain.
In the palm of my hand, I hold an eleven.
After two seconds, I am standing in a mild panic. By three seconds, the only way I can continue is to grasp my left wrist with my right hand. I command my right hand, well clear of the candle's flame, to hold my left hand in place.
At five seconds, I pull away from the candle. I plunge my hand into the bowl of snow. The pain drops from somewhere near eleven to something manageable. A nine. A seven. A three. I sit down. I pant. I stare at the candle flame. And I realize that today is the day I begin to understand heat.
H
eat, from a human perspective, is more than just a matter of high temperatures in the shade. Two doctors working in the 1950s watched marines in the heat and humidity of Paris Island, North Carolina. They wrote of “disabling heat” at temperatures in the eighties. Young marines wore fatigues, helmets, cartridge belts, bayonets in scabbards, and boots. They carried full canteens, rifles, entrenching tools, and twenty-two-pound packs. Marching in the sun, running in the sun, low-crawling in the sun, digging foxholes in the sun, they complained of cramps and exhaustion. Young marines occasionally collapsed.
The doctors saw that temperature alone could not predict heatstroke and heat exhaustion as well as what became known as the Wet Bulb Globe Temperature. When a marine recruit suffered in the heat, he also suffered in the humidity and the sun. The Wet Bulb Globe Temperature combined the air temperature in the shade, the air temperature in the sun, and something called the “natural wet-bulb temperature.” The air temperature in the sun is measured with a black globe thermometer—a thermometer inside a blackened sphere of copper, left in the sun. The natural wet-bulb temperature comes from a thermometer with its bulb covered by a moistened sock. As the moisture evaporates, it removes heat. Increase the humidity and decrease the rate of evaporation, and in turn increase the wet-bulb temperature. Add a breeze to increase the evaporation rate, and in turn decrease the wet-bulb temperature.
Using the Wet Bulb Globe Temperature, the doctors figured out what every child knows: humid days feel hotter than dry days, and shaded ground is cooler than sun-bathed ground.
A flag system formalized their findings. A yellow flag warned against exercise in the sun: “Instruction involving strenuous physical exertion, such as an infantry drill, crawling on the ground, or bayonet training, will be given only in the shade.” A red flag meant it was too hot to work: “During the time a red flag is displayed, recruits will be given only that type of instruction which can be given to men standing or sitting in the shade.” Later a black flag was added: “All nonessential physical activity will be halted for all units.”

My companion and I fly south to Las Vegas and rent a car. She drives while I stare at the sun-burdened landscape. Warning signs mark the entrance to Death Valley itself: “
DANGER: EXTREME HEAT
” and “
LIMIT OUTDOOR ACTIVITY TO SHORT WALKS
.” If there were flags here, they would be black.
We park near the lowest point in Death Valley and walk into a canyon. We carry a gallon jug filled with water. We walk between vertical walls of white and yellow mudstone that leave the floor of the canyon in almost constant shade, and a breeze funnels between the walls. Still, the heat feels dangerous. It threatens us as though it has intentions, as though it wants to stifle breathing. The plan is a cautious walk, half an hour at the most, a stroll to get a feel for the land.
But that is not possible. The land lures us. This is a place that we must experience. Where we had planned to turn around, at the end of the canyon, we find a trail leading through badlands. We are in open sun now, in a place where the black globe thermometer temperature might approach the boiling point, but we are saved by a combination of low humidity and breeze and stupidity. We move up and down across low but steep hills that may once have been sand dunes. To our left, a red cliff towers. To our right, scorched hills stretch into the distance.
As a measure of the brutality of this land, cactus does not grow here. This place is too dry and too hot for cactus. But we come across occasional bushes, mostly desert holly, which is not a true holly at all. It is a chenopod, with leaves shaped like those of a holly, but paler.
The trail branches and then branches again. We find a sign with arrows that should point right and left, but the sign is lying on the ground, pointing nowhere. We find another sign, this one warning of abandoned borax mines, and we follow it, wondering what a borax mine might look like.
After an hour, our gallon of water has become a half gallon. The breeze has died.
I begin to wish that we had told someone where we were going.
Here is the irony of Death Valley: Its desiccated beauty was carved by water. As recently as 1976, a sudden flood destroyed a road that ran near here. Everywhere signs of erosion by flowing water prevail. This is not gentle erosion over time. This is sudden erosion, driven by water that appears from rare cloudbursts to rush through channels, carrying away dirt and stones and boulders and leaving behind dry channels called arroyos. We walk down an arroyo now, comforted by occasional footprints as we move through our second hour.
With my infrared thermometer, I shoot a temperature in a tiny patch of shade: 112 degrees. I shoot another in the sun: 124.
In this heat, without water, with little shade, it is possible to die in as little as seven hours. Death would come from a combination of dehydration, heat exhaustion, and heatstroke. The blood’s plasma volume would drop. The blood and the water bathing tissues and residing in bones would grow increasingly salty. Firing of nerve cells would become irregular as membranes changed and ion pumps—cellular pumps that maintain the interior chemistry of cells—failed.
When water for sweating is exhausted, when sweating stops, core temperature rises. The victim succumbs to heatstroke. As the core temperature reaches 107 degrees, the lining of the intestine fails, releasing fatty sugars into the bloodstream and triggering what amounts to septic shock. Among other things, the brain grows ill. In an end such as this, death comes from systemic failure, from a syndrome expressing in a hundred different ways the fact that the body no longer functions. A mere eight- or nine-degree increase in core temperature kills without mercy.
We move slowly, resting often, drinking in the landscape. The arroyo seems to loop back to the paved road where we parked our car, a path that will let us see the desert without backtracking. We find slivers of shade against pale cliffs of tortured mudstone that rise up from the arroyo. Ripples decorate the mudstone walls, more signs of flowing water. In places, mud liquefied by long-ago rains settled and was baked into flat shelves that make perfect benches in the shade. When we sit, the heat of the ground moves up through the seat of the pants.
We are down to a single quart of water. We worry that the arroyo may not lead to the road after all. We agree to turn around and retrace our path before drinking again, but then we press on.
A dry creek bed, known in this part of the country as a dry wash, intersects the arroyo’s right bank, forming a confluence of dusty channels. Above it, I see a mine shaft. A mound of mine tailings stands just outside the opening of the shaft. The dry wash is steep, and the mine shaft is up an even steeper incline above the wash, a hundred yards away under full sun. But I want to see the shaft.
The shaft’s opening is seven feet tall and three feet wide, the shape of an arching doorway, a tunnel entrance standing dark but surrounded by sunlit pale rock. The shaft itself runs straight back into the hill. I feel my way forward, blind in the shadows, shuffling my feet, and I am surprised when the tunnel ends. I stand in what feels like total darkness perhaps twenty-five feet from the entrance. Here, in the back of the tunnel, the heat is gone. The temperature is below ninety degrees. I stand in the darkness, savoring cool air.
Back outside, nearby, I find another tunnel. This one is cut through a hill, with openings on both sides, a would-be breezeway but for the total absence of air movement. In the middle, halfway between the openings, two rusted iron bed frames sit end to end. Miners must have slept here. Where they found water is not clear. Rainfall here averages two inches per year. Some years, no rain falls at all.
When they were not sleeping, the miners were busy. The hills around their breezeway are rotten with tunnels. In one, I find a half cup of guano piled on the floor. In another, I find distinct lines of salt, white crystals in the tunnel’s wall. I lick the rock. It is not sodium chloride. It could be sodium borate. The miners sold the borate salts, a compound of boron used in fertilizers, insecticides, detergents, wood preservatives, and cement. Added to glass, boron prevents cracking from heat. Added to some fabrics, it acts as a flame retardant. Added to iron smelters, it removes unwanted oxides. Added to nuclear reactor shields, it blocks neutrons. Added to humans in large-enough quantities, it causes headaches, vomiting, and eventually liver cancer.
The big borax operations in Death Valley were out near Furnace Creek, several miles from here, in the 1880s. Near Furnace Creek, miners dug for ore rather than the almost pure salts. The borax ore was boiled in vats with water and carbonated soda, and the solution was cooled in tanks wrapped in wet felt. Water evaporating from the felt sped the cooling process. When cooled to 120 degrees, borax salts crystallized.
Over a few decades, twenty million pounds of borax salts were hauled out in twenty-mule trains—two wagons full of borax and a water tank pulled by twenty mules or, more commonly, eighteen mules and two horses. The wagons had spoked wheels seven feet in diameter.
The distance to the nearest railroad: 165 miles.
During summers the cooling vats could not be cooled to 120 degrees. The borax ore miners took their summers off.
From a 1907 Death Valley newspaper advertisement: “Would you enjoy a trip to Hell? You might enjoy a trip to Death Valley! It has all the advantages of Hell without the inconveniences.”
We make our way down the wash, back into the arroyo. I shoot the temperature of a rock in the sun: 129 degrees. We drink all but the last two tablespoons of our water, now as warm as tea, but abandon our plan to turn back. The road, we think, is just ahead, just around the next turn. My companion’s face is flushed. A heat rash has blossomed on her legs. It has been three hours since we left our car.
The badlands that we move through are vegetated to the same extent that an asphalt parking lot is vegetated. We see, at times, the tracks of lizards, but never the lizards themselves. In places a black crust covers the white cliffs. Elsewhere gray-green veins run through the rocks.
The arroyo narrows into a gorge with walls too steep to climb. Now we walk in shade. We climb down rock ledges in the gorge, losing elevation. I feel ill, but the symptoms are not concrete. It is a general malaise, a feeling of exhaustion mixed with impatience.
At four hours, the arroyo returns us to pavement. We find our car. I shoot temperatures. The asphalt road registers 148 degrees. Inside the car, the driver’s seat registers 180 degrees.

Antoine Lavoisier, French nobleman and brilliant chemist, promoter of the caloric theory of heat, the man who dephlogisticated the phlogiston theory of heat, realized that animals burned food in a reaction identical to that in which woodstoves burned firewood and in which lamps burned whale oil. “La respiration,” Lavoisier wrote, “est donc une combustion.” In English: “Breathing is thus a combustion.”